A smoke-free future and beyond

Our researchers have published images of solid particles from cigarette smoke as small as tens of nanometers, and they’ve measured nanograms of chemicals. They’ve collected terabytes of data, for which our scientists have developed high-performance computing infrastructure to store and analyze that data. And, with 984 randomized participants enrolled in the 6-month study, they’ve conducted what is likely still the largest study of its kind to date on the topic of tobacco. We’ve even published a book on the toxicological evaluation of electronic nicotine delivery products.
In short, PMI has an outstanding research facility and excellent capabilities across a range of scientific fields. So why wouldn’t we put those facilities and capabilities to good use?
We already apply ourselves to our smoke-free product research and assessment, working to transition away from cigarettes and toward smoke-free products.* That’s what the Cube was built for. But we also do much more than that, contributing more broadly to the fields of research in which we work.
Here are a few examples of the research PMI currently conducts, both alone and in collaboration with other researchers, and an explanation of how that work could also impact research beyond smoke-free products.
Understanding the airway
Considering the fact that smoke-free product aerosols are inhaled into the lungs, it is no surprise that our scientists are experts at modeling and understanding interactions between aerosols and the human airway — the path through which air reaches the lungs. This research is clearly important to understanding our smoke-free products, but it can also be informative for scientists working on inhalable therapeutics.
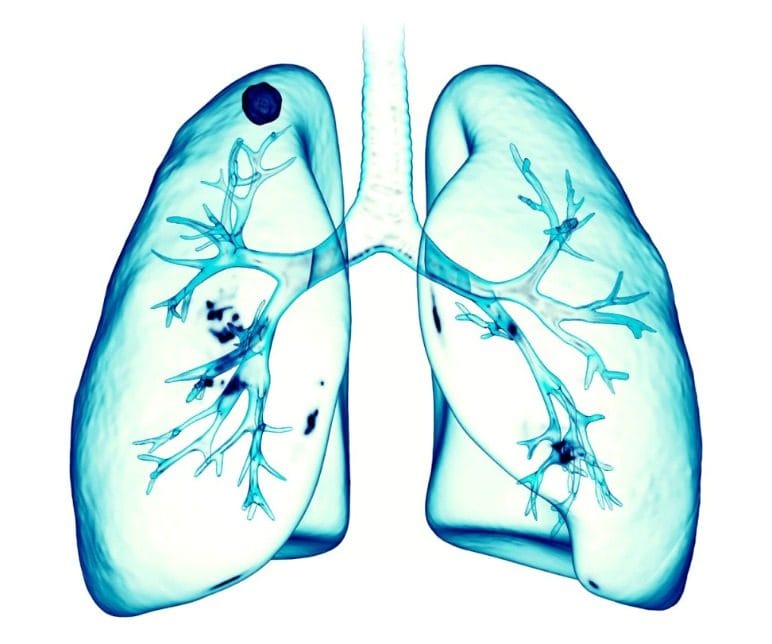
In February of 2020, we were part of a team that published the research showing how models of the airway can be personalized to an individual’s unique airway shape. Age, gender, weight, fitness, health, and disease status can all impact the shape and functioning of an individual’s airway. This publication details a model that can be used for person specific simulations of how air is breathed in, gases are exchanged, and an aerosol is deposited along the airway. Information like this could help scientists who are working on therapeutics better understand how pharmaceuticals are delivered into the body, their efficacy, and determine or predict possible adverse effects before they happen.
More recently, PMI scientists were part of a collaboration to develop three-dimensional organotypic cell cultures of the human lung from multiple donors, creating a cell culture with approximately the shape and function of real human lung tissue. These cell cultures varied from donor to donor in their shape and their response to stimulation, but they were able to work and metabolize similarly.
Building on these avenues of research, PMI scientists have sought to develop a new system that can simulate exposure to various inhaled substances. The independent holistic air-liquid exposure system (InHALES) simulates the human airway in both shape and function, delivering aerosol doses to cell cultures. The system, like the human respiratory tract, can actively breathe, operate medical inhalers, or take puffs from tobacco products.
Advancing toxicology methods
PMI is at the forefront of systems toxicology, using systems biology and applying it to toxicology. This branch of science aims to quantitatively understand, model, and predict the response of cells to external stimuli and can be expanded to assess systems and organs. In our product assessment, we use systems toxicology to build a detailed understanding of the mechanisms driving the biological response to toxicant exposure. This approach and these methods can be used to assess the risks of chemicals, drugs, and consumer products.
Predicting the impacts of the exposure to a new compound is a fundamental challenge in biomedicine that systems toxicology can help with. This prediction means identifying both the intended and non-intended effects of the compound. In one review, we explain why systems toxicology is a powerful approach to predict the likelihood of undesired drug effects and begin to outline the mechanisms leading to these effects. In systems toxicology, scientists combine the power of technologies that allow rapid aggregation of large quantities of data with advanced computational approaches that use complex network models with standard endpoints from preclinical and clinical studies. Doing this allows for a system-wide evaluation of the effects a drug can have on the biological system.
The effects of drugs and chemicals on the system can be measured through biomarkers, which are chemicals or other signals in the body that can indicate something about the body’s response. For example, exposure to a chemical or drug, development of a disease, or how far along a given disease might be. These could all be quantified to some extent based on biomarker measurement.
Biomarkers can be especially important for detecting and monitoring the progression of many diseases. Because many of the available biomarkers are often not specific enough, better biomarkers are needed to improve disease risk assessment and monitoring. In this review, we discuss research findings on lipid biomarkers and the possible connection between these biomarkers and cardiovascular disease, chronic obstructive pulmonary disease, and aging. We also stress the need for improved quantification, method standardization, and establishing reference values to more efficiently translate research findings into the clinical practice so that newly identified biomarkers can be put to practical use.
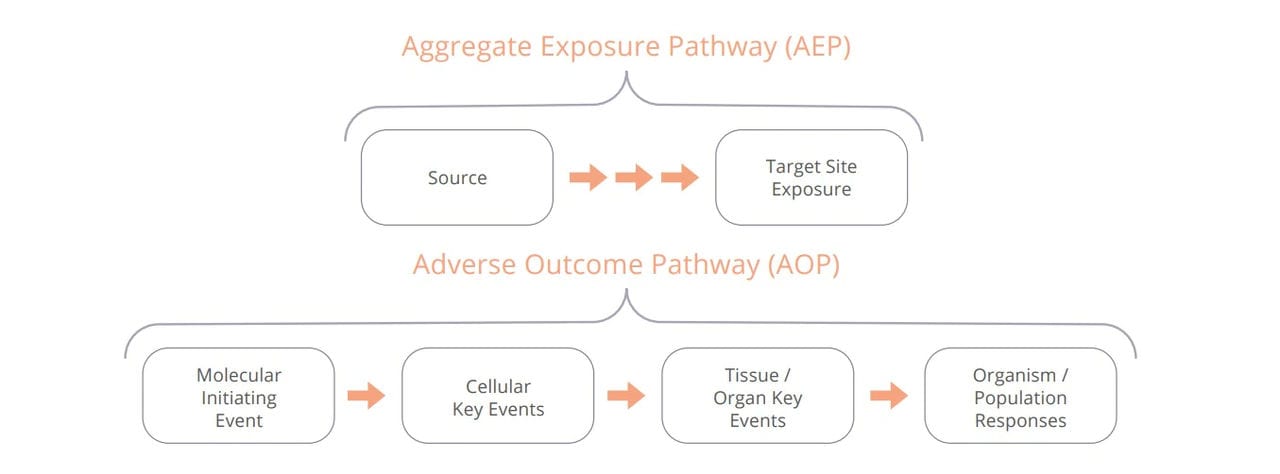
Establishing a pathway from exposure to response. An AOP begins when the exposure initiates the first event in the pathway. An AOP contains one or more key events inside the body, and ultimately results in the organism level or population level response.
Reducing animal studies in research
Organ-on-a-chip technologies, also called microphysiological systems, represent promising tools to advance patient benefit and reduce animal testing, because they can mimic the human biology in vitro by allowing multiple “organs” to work together. But there are still many hurdles to their wide use in industry and acceptance by regulators. A team of 46 experts, including PMI scientists, worked together to analyze the current life-cycle of organ-on-a-chip-based assays. In the resulting extensive report, the authors give recommendations and a roadmap towards regulatory acceptance of models based on organs-on-a-chip systems. Such regulatory acceptance would be to the patients’ benefit and would reduce the use of laboratory animals in the drug development process.
Scientists can also minimize the use of animals in research when they have a good understanding of the mechanisms that lead to disease or a negative reaction to a chemical. Adverse Outcome Pathways (AOPs) describe a series of biological events that lead to adverse or undesired effects. Developing AOPs can help identify and address gaps in our understanding of disease development or chemical exposure. This lets scientists identify key events involved in causing toxicity and then optimize non-animal approaches that can be used to investigate them.
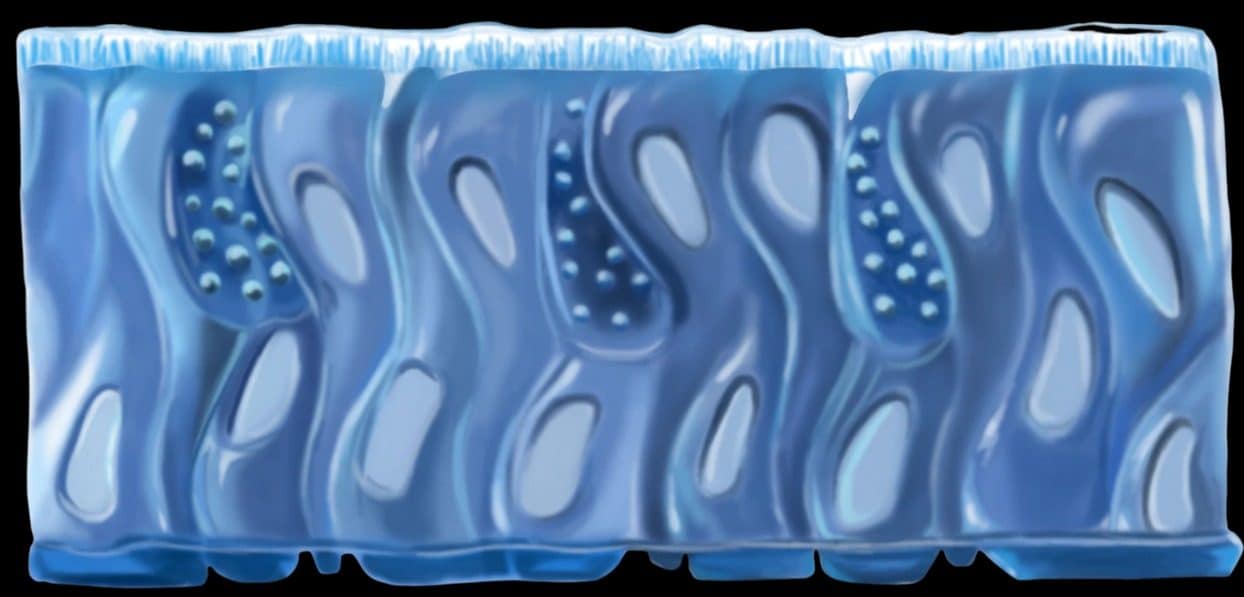
An artist’s rendering of the human bronchial cells used in a lung/liver-on-a-chip device. Such devices represent promising tools to reduce animal testing.
In a 2016 workshop attended by government agencies, industry, academics, and non-governmental organizations, experts developed a strategy to establish confidence in non-animal approaches for the assessment of acute inhalation toxicity. This 2018 paper details the outcome of that workshop. It describes the AOPs and the toolbox of in vitro and in silico models that can be used to assess the causal chain of events leading to toxicity following inhalation exposure. As part of the workshop, the participants developed a decision tree to guide scientists toward designing their studies with strategic consideration to minimize animal use where possible. This decision tree can also facilitate standardization of non-animal testing approaches.
Pitching in during a pandemic
Like many people around the world, our researchers spent time in spring 2020 contemplating the question: What can I do to help my neighbors during a global pandemic? Our researchers came up with many ways to help, and they continue to generate new initiatives that make a difference.
As many people experienced around the beginning of March 2020, there was a hand sanitizer shortage. That was the case in Neuchâtel, the location of our research facility, the Cube. On Thursday, 19th of March 2020, Switzerland’s federal office of public health, the FOPH, suspended the requirement for authorization to produce alcohol-based disinfectant so that it could be made more readily, in response to the crisis. One day after the requirement was lifted, PMI scientists had produced 450 bottles, all with labeling in accordance with national guidelines. The next Monday, we were already delivering the bottles to cantonal authorities.
In two months, 400 liters of hand sanitizer were produced in the Cube, 300 liters of which were donated to cantonal authorities for hospitals, to homes, and to the Neuchâtel Center of Psychiatry (Centre Neuchâtelois de Psychiatrie). Cube scientists also advised our colleagues in PMI factories around the world how to make the hand sanitizer, resulting in tens of thousands of bottles donated globally.
PMI scientists have also assisted local efforts to combat COVID-19 by supporting testing efforts beginning in March 2020. As part of multiple collaborations with area hospitals, PMI scientists have developed sensitive test methods for the virus, performed and collected nasopharyngeal swab collection and also rapid antigen tests, and analyzed hundreds of test samples.
A lasting positive impact
Whether they are conducting research on our smoke-free products or branching out to other topics like new initiatives such as botanicals or respiratory drug delivery, our researchers apply state-of-the-art methods and standards for study development, data collection, and analysis. As is often the case in science, the approaches we’ve used and results we’ve collected along the path of our assessment program can be useful to researchers beyond the tobacco industry. Our scientists are proud to be working toward delivering a smoke-free future, and by now it’s clear that their work can have an even broader positive impact through the sharing of our science. That’s an opportunity we’re not willing to pass up.
* Smoke-free products are not risk free and contain nicotine which is addictive

Наш научный журнал Scientific Update
Наш научный журнал Scientific Update посвящен исследованиям и разработкам ФМИ, ключевым исследованиям, регулированию и многому другому. Ознакомьтесь с последним выпуском или прочитайте статьи онлайн.