An alternative to animal use in risk assessment of tobacco products
Organ-on-a-chip systems are promising tools that can transform science, medicine, and patients’ lives. They have the potential to improve disease modeling, understanding of human disease physiology, and toxicological assessment. The use of human cells in organ-on-a-chip systems will improve the accuracy prediction of exposure effects in humans and reduce animal testing. However, these advances have also brought new challenges, such as slow industrial adoption and regulatory acceptance. A team of 46 experts including Philip Morris International (PMI) Scientists jointly analyzed the current life cycle of organ-on-a-chip-based assays and gave recommendations on addressing those barriers, in an extensive report.
Organ-on-a-chip technologies are microfluidic devices containing living engineered tissue or organ substructures that emulate the biology of entire organs or organ systems in vitro. The application of a fluid flow makes them dynamic and superior to conventional, static cell culture systems; the flow offers the possibility of generating biomolecular gradients and mechanical cues that mimic the complexity and physiological response of entire organs or organ systems. The use of cell models that are more biologically relevant to human biology in organ-on-a-chip-technologies could allow the technologies to be more predictive in understanding human biology and disease than laboratory animals and conventional in vitro cultures. Thus, organ-on-chip technologies are promising alternatives to animal testing.
PMI uses organ-on-a-chip technology to minimize animal testing
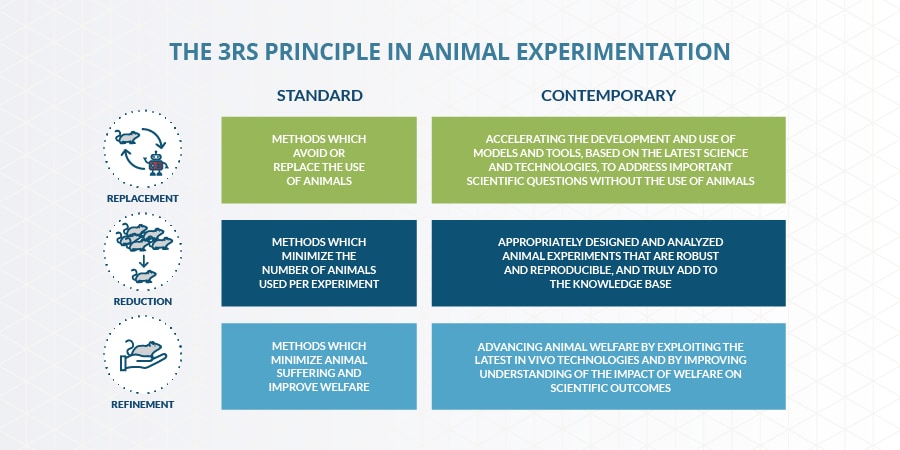
At PMI, under the perspective of the 3R principles aimed at “replacing, reducing, and refining” animal testing, we increasingly integrate organ-on-a-chip technology in our product assessment activity. For example, we used a 3D vasculature-on-a-chip model to compare the impact of exposure to the Tobacco Heating System (THS), a modified risk tobacco product, to the exposure to the 3R4F reference cigarette, on the adhesion of monocytic cells to endothelial microvessels.
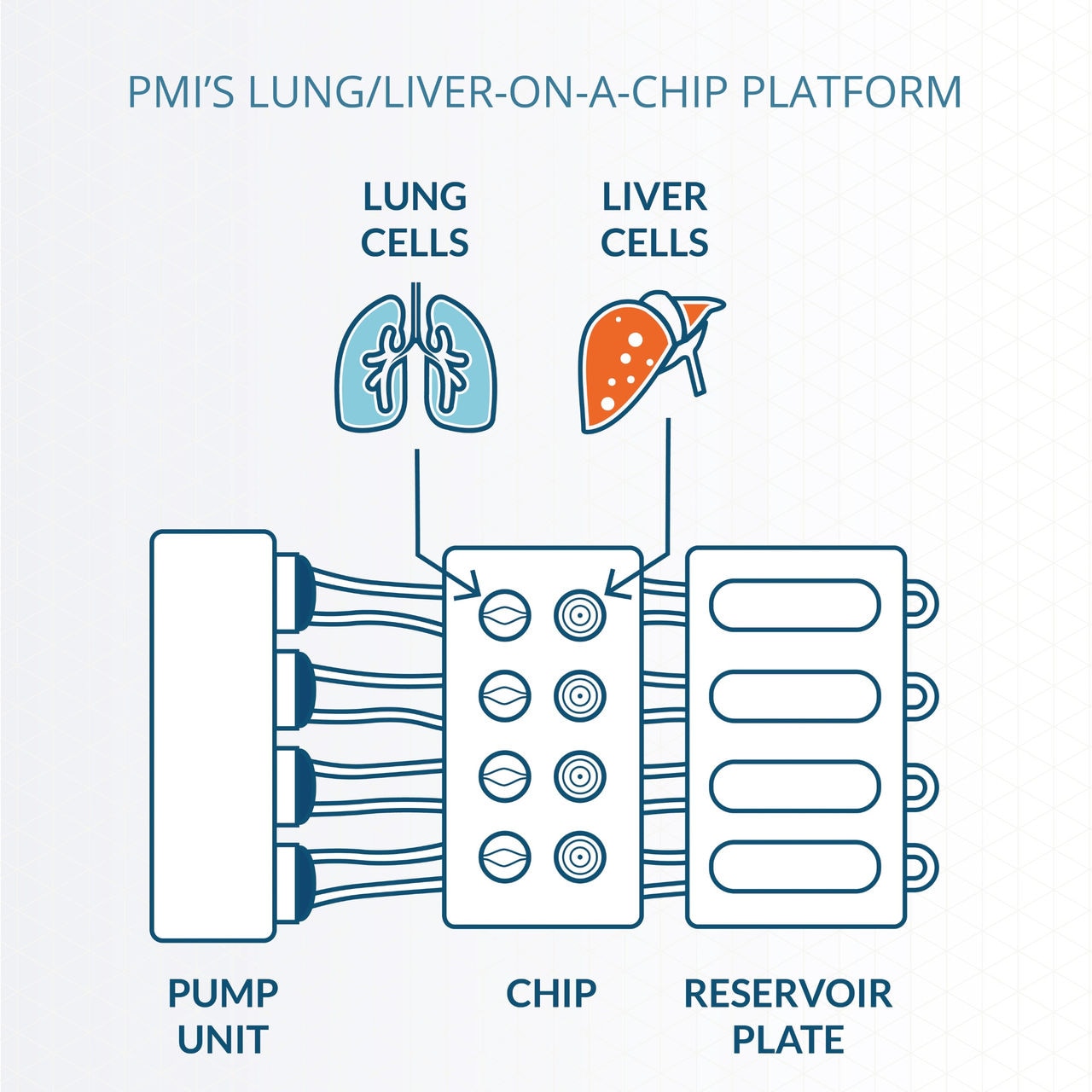
We also continuously develop organ-on-a-chip technologies to evaluate the potential toxicity of inhaled compounds better. Recently, we developed a lung/liver-on-a-chip platform, connecting a human lung epithelial model with a human liver spheroid model in a single circuit. The incorporation of a liver model aims to accurately test not only the potential toxicity of the inhaled parent compounds but also that of their metabolites, which are generated by cytochrome P450 (CYP) enzymes expressed in the liver.
PMI participated in an international workshop on organ-on-a-chip technology
In June 2019, the Center for Alternatives to Animal Testing (CAAT) Europe sponsored a three-day workshop on organ-on-a-chip technology. Forty-six leading experts from the four relevant stakeholder groups, namely academia, the supplier industry, end users (pharmaceutical and consumer products industries), and regulatory agencies, worked together to analyze the life cycle of organ-on-a-chip based assays in order to:
- identify hurdles for their adoption and regulatory acceptance,
- and provide recommendations on how to overcome these hurdles.
The outcome of this collaborative work is available in the form of a report, which is published in a peer-reviewed scientific journal. Two PMI scientists working on organ-on-a-chip technology in-house, Julia Hoeng and Anita Iskandar, attended the workshop as consumer product industry experts and contributed to the report. The common objective of the workshop was to improve the acceptance and use of organ-on-a-chip-based assays for regulatory submission.

Screenshot of the report on organ-on-a-chip technology, published in the journal Altex. The report is available for free at the publisher’s website.
The experts recognize that organ-on-a-chip technology, which started in academia more than 15 years ago, has progressed incredibly. The report highlights the implementation of the technology for clinically relevant research. Each stakeholder is active in the research and development of organ-on-a-chip technology individually. However, there have also been joint initiatives to collaborate further in the development efforts of tissue chip, such as the IQ Consortium and the Human Organ and Disease Model Technologies.
Despite the advancement, organ-on-a-chip-based assays are still not well established within the discovery and development of drug and consumer products. The challenges and hurdles are summarized in the figure below:
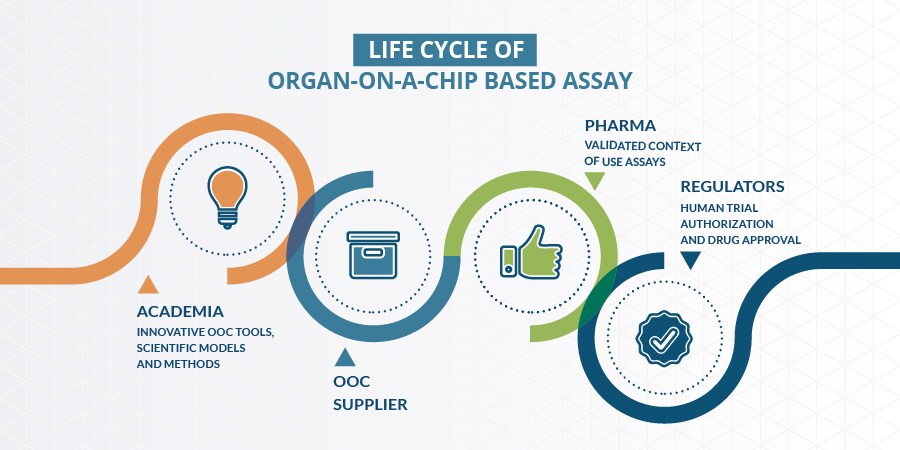
The four stakeholder groups involved in the life cycle of organ-on-a-chip (OOC) based assays. Adapted from Marx et al, 2020.
The main hurdles commonly identified by the experts are the communication gap among stakeholder groups and the lack of qualification of assays, leading to “regulatory acceptance dilemma”: The end-users reason that regulators do not clearly communicate the acceptance of data generated using organ-on-a-chip technology and do not provide guidance on which data is needed; the regulators, in turn, assert that the end-users do not provide robust data (or very limited); therefore in the end, experience and confidence on the data generated from organ-on-a-chip technology cannot be gained. This challenge applies not only to the pharmaceutical industry but also to tobacco industry for the development and assessment of candidate modified risk tobacco products.
What do experts recommend to improve the situation?
The experts recommend several immediate actions to improve the adoption of organ-on-chip technologies in regulatory applications:
- To improve the communication gap: To extend the existing joint initiatives, such as the EUROoCS and Japanese AMED, and to find a global organ-on-a-chip technology society, which would host congresses, organize training, and provide funding to address research gaps.
- To support the qualification of organ-on-a-chip-based assays: To establish centers for the evaluation and validation of organ-on-chip technologies that involve all main stakeholders (i.e., academia, suppliers, end-users, and regulatory agencies).
- To address the regulatory acceptance dilemma: To encourage data sharing from end-users or to make the data publicly available wherever possible and to develop guidance documents by regulators.
The immediate recommendations and a roadmap of actions for the next 15 years are provided in more detail in the report. All participants of the workshop are confident that in the long run, organ-on-a-chip-based assays will reach the necessary level of qualification and regulatory acceptance, leading to a tangible impact on patient benefit and animal welfare.