What are the differences between smoke and aerosol?
Learn more about differences between smoke and aerosols with Dr. Markus Nordlund, expert in aerosol physics and combustion science.
The harms of cigarette smoking are well known. Smoking-related harm and disease are caused by long-term exposure to the toxicants in cigarette smoke. For current smokers, the best step they can take to reduce their risk of harm is to quit tobacco and nicotine use altogether. But the fact is that not every smoker quits.
As part of the tobacco harm reduction approach, alternatives to cigarettes have been developed such as the THS. THS does exactly what its name suggests: it heats tobacco instead of burning it. That is the fundamental difference between THS and cigarettes. By not burning tobacco, we have shown that THS doesn’t create smoke, but instead, a non-smoke aerosol which, while still not risk free, contains significantly fewer and lower levels of harmful chemicals compared with cigarette smoke.
Smoke, according to the scientific consensus, is an aerosol formed during combustion and high-temperature pyrolysis which contains liquid and solid particles also known as particulate matter as well as gases.
The particulate matter found in smoke is formed when products of combustion and high-temperature pyrolysis reach high enough concentration (specifically, they reach supersaturation) to nucleate via condensation or interact with each other to form particles. In particular, the carbon-based solid particles found in smoke (also known as soot) are formed from high concentrations of certain chemicals acting as precursors, such as polycyclic aromatic hydrocarbons, that are produced only at the high temperatures associated with combustion.
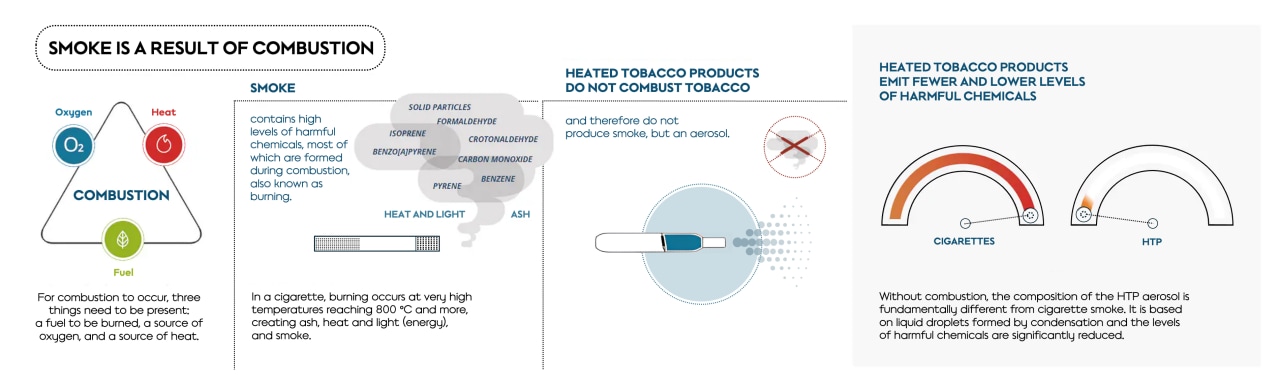
Cigarette smoke is a product of combustion, which is defined as a chemical process of oxidation that occurs at a rate fast enough to produce heat and usually light in the form of either a glow or flame. Combustion also includes both complete and incomplete (partial) combustion processes, such as smoldering (flameless) and flaming combustion.
The combustion of a cigarette takes place after ignition, when temperatures in a cigarette exceed about 400 °C. It then becomes self-sustaining as long as the exothermic (heat-generating) oxidation reaction is sufficiently strong to overcome heat losses and endothermic (heat consuming) processes, such as vaporization and endothermic thermal decomposition.
During combustion, particulate matters are formed when combustion products reach high enough concentration to nucleate via condensation, or when they interact with each other to form liquid particulate matter (droplets) and solid particulate matter such as soot.
Cigarette smoke is a complex and dynamic chemical mixture that has been well characterized, with billions of carbon-based solid particles (soot) and more than 6,000 constituents identified. Within this complex mixture, about 100 constituents have been associated with smoking-related disease by public health authorities. These constituents are also known as harmful and potentially harmful constituents (HPHCs).
While smoke is an aerosol, not all aerosols are smoke. The aerosol from THS contains droplets which are not formed from the condensation of byproducts of combustion or pyrolysis. Instead, these droplets are generated when glycerol—added to the tobacco material during processing as an aerosol former—is vaporized and reaches supersaturation, leading to its condensation during the cooling phase and the formation of nuclei, onto which more glycerol, as well as water, nicotine, and other constituents can condense to form liquid aerosol droplets.
Thus, aerosols formed from THS are not smoke as they are very different in terms of origin and chemical and physical composition to the smoke formed from the combustion and associated high-temperature pyrolysis products generated from the burning of tobacco. Furthermore, we have conducted several research studies substantiating that no combustion of the tobacco material occurs in our THS and that our THS aerosol is liquid-based and is not smoke.
Actually, the aerosol generation process in THS is equivalent to the aerosol generation process in most e-vapor products, for which aerosol formers (glycerol and propylene glycol) in the e-liquid are vaporized during heating and are subsequently cooled down to form liquid aerosol droplets.
Learn more about differences between smoke and aerosols with Dr. Markus Nordlund, expert in aerosol physics and combustion science.
THS doesn’t need oxygen to work because there is no combustion (oxidative process) as the tobacco material is heated to temperatures below ignition. In fact, the tobacco material within THS undergoes processes such as drying and vaporization, as well as thermal decomposition (torrefaction and low-temperature pyrolysis) which are not associated with combustion, either complete or incomplete.
Furthermore, the comparison of the chemical composition of THS aerosol generated in oxidative (air) and non-oxidative (nitrogen) environments indicates that oxygen—necessary for combustion to happen—does not play a major role in the thermal decomposition of the tobacco material in THS or the aerosol formation.
The absence of combustion in THS has been substantiated by scientific evidence and has been verified by third-party scientific experts in numerous countries as well as by independent research organizations.
By eliminating combustion in our tobacco-containing smoke-free products, we aim to drastically reduce the formation of HPHCs and to generate a liquid-based aerosol without the billions of carbon-based solid particles being a hallmark of smoke. Our principle is to heat tobacco to temperatures low enough to avoid ignition and burning. This allows nicotine and flavors to be released from the tobacco, while generating significantly lower levels of HPHCs compared with cigarette smoke.
It is to note however, that THS are not risk-free and contain nicotine which is addictive.
In smoke, billions of carbon-based solid particles or soot, are typically produced during combustion, and inhaling them has been shown to trigger inflammation and to cause lung and cardiovascular disease. Our THS has been proven not to produce these carbon-based solid particles by our research as well as by numerous peer-reviewed publications by various research groups. So, there are no solid particles in the aerosol produced by THS.
As we have seen throughout this article, smoke is a product of combustion or high-temperature pyrolysis. And the smoke generated by these processes contains billions of carbon-based solid particles (soot) as well as high levels and numbers of harmful chemicals.
Smoke-free products, such as THS, do not combust tobacco and therefore do not generate smoke.
* Commercialized as IQOS

The Scientific Update magazine is focused on PMI's research and development efforts, milestone studies, industry regulations, and more. View the latest issue, or read the articles online.