Historic decision made as IQOS Tobacco Heating System is authorized under U.S. MRTP pathway
U.S. FDA recognizes fundamental differences between IQOS system and cigarettes
The U.S. FDA announced on July 7, 2020 authorization to market the IQOS Tobacco Heating System with reduced exposure Information. This is the second set of products ever to be authorized as Modified Risk Tobacco Products (MRTPs), and the first electronic nicotine product to receive such authorization.
The FDA’s decision provides an important example of regulation of smoke-free alternatives to differentiate them from combusted cigarettes in order to promote the public health. The U.S. legal framework and FDA policy recognizes that tobacco products exist on a continuum of risk, with combusted cigarettes being the most harmful, and provides a regulatory framework for manufacturers to inform consumers about products with different risk profiles. The decision clearly differentiates our leading HTP from combusted cigarettes and allows American men and women who smoke to receive information about a product that is a better choice than continuing to smoke cigarettes. But first, a quick review on how we got to where we are now.
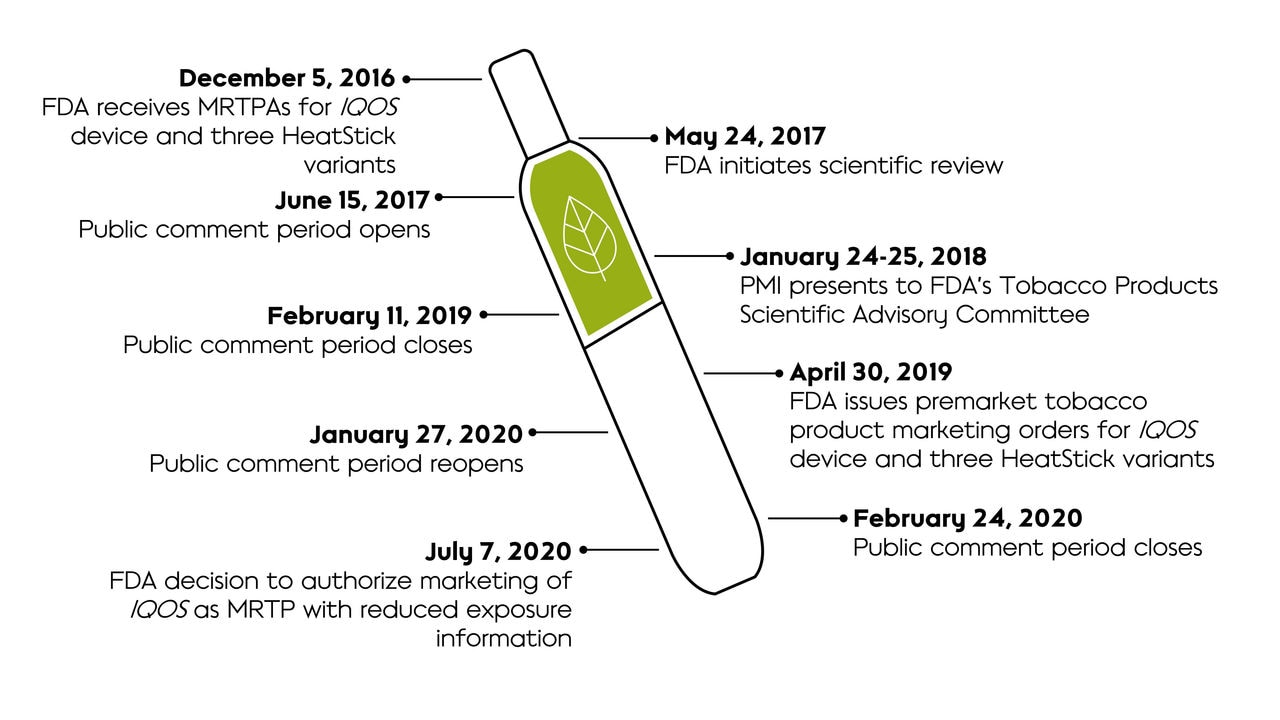
Timeline of the MRTP review process
The MRTP application review process began with several meetings between Philip Morris International (PMI) and the FDA to discuss plans to submit an MRTP application. With the initial submission of the application in December 2016, FDA then determined whether the product fell under the jurisdiction of the Center for Tobacco Products and that legal requirements for the application were met. With the application in hand, FDA reviewed the filing to ensure the application contained all the necessary content, and then began scientific review of the application.
PMI’s requested MRTP claims
The FDA has authorized the marketing of IQOS Tobacco Heating System in the U.S. as an MRTP with Reduced Exposure Information. The IQOS system is the name under which our Tobacco Heating System (THS) is marketed. The agency found that the issuance of the modified risk tobacco product orders with reduced exposure information would be “appropriate to promote the public health and is expected to benefit the health of the population as a whole.” The FDA also concluded that the totality of evidence presented suggests that a measurable and substantial reduction in morbidity or mortality among individual tobacco users is reasonably likely to be established in subsequent studies.
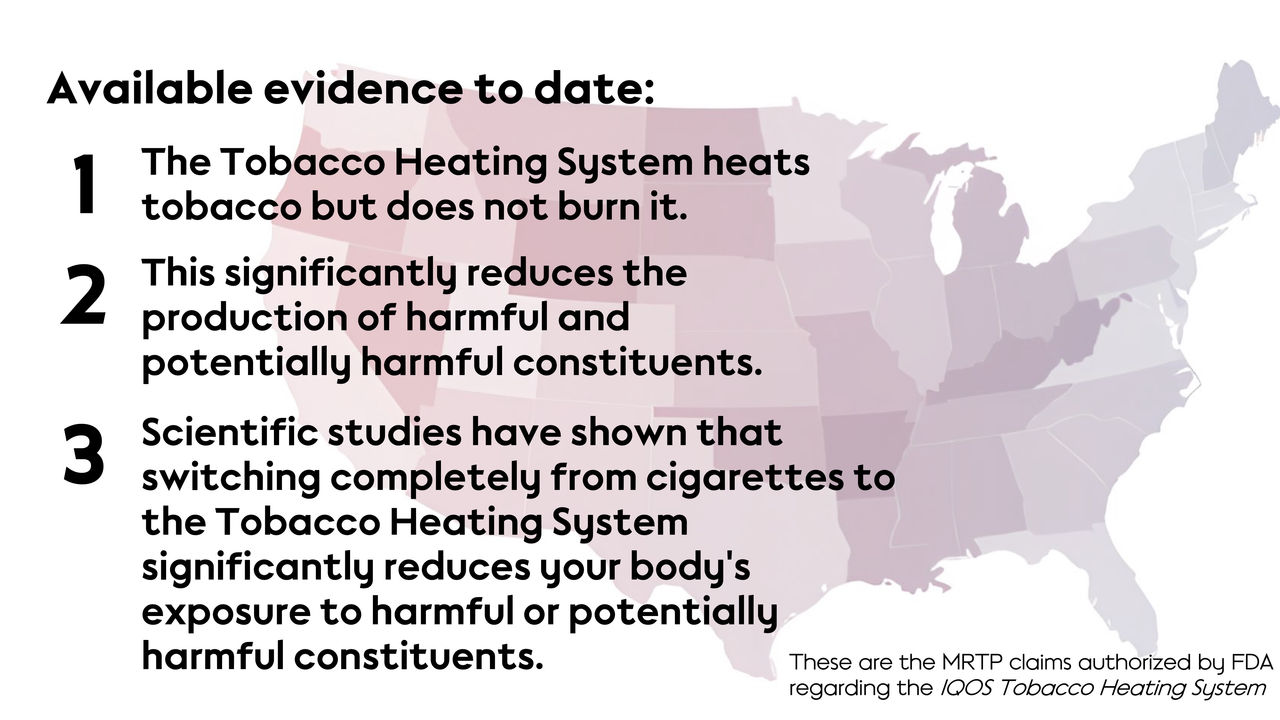
There are two types of modified risk orders the FDA is authorized by statute to issue: a “risk modification” order or an “exposure modification” order. PMI had requested both types of orders for THS. In its Technical Project Lead (TPL) summary review of the decision, the FDA determined that the evidence that has been submitted by PMI did not support issuing risk modification orders at this time—according to the FDA's interpretation of the Tobacco Control Act standard for issuance of such orders—but that the evidence submitted did support issuing exposure modification orders for these products. This determination included a finding that issuance of the exposure modification orders is appropriate to promote public health and is expected to benefit the health of the population as a whole, taking into account both users of tobacco products and persons who do not currently use tobacco products.
In particular, the agency determined that PMI demonstrated that THS heats tobacco and does not burn it, and that this significantly reduces the production of harmful and potentially harmful constituents (HPHCs) compared with cigarette smoke. The FDA also concluded that the totality of evidence presented suggests that a measurable and substantial reduction in morbidity or mortality among individual tobacco users is reasonably likely to be established in subsequent studies.
While we are convinced that the totality of evidence demonstrates that switching completely to THS presents less risk of harm compared to continued smoking, we will continue to work closely with the agency to determine what information would allow the FDA to conclude that the specific and statutory requirements in the U.S. are met, and therefore to authorize us to communicate reduced risk or harm information.
This decision follows the agency’s April 2019 authorization of the marketing of the IQOS Tobacco Heating System as “appropriate for the protection of public health” pursuant to the Premarket Tobacco Product Application (PMTA) pathway.
The science behind reduced exposure
The high temperature process of burning is known to create thousands of chemicals in smoke, many of which are harmful or potentially harmful. By not burning tobacco in THS, we significantly reduce, in some cases even eliminate, harmful chemicals compared with cigarettes. Using clinical studies, we confirmed that the reduced formation of harmful chemicals translated to reduced exposure among those who switched from cigarettes to THS. The FDA reviewed this evidence, together with research conducted by independent scientists, and found it to be sufficient to allow this information to be communicated to those adult men and women in the U.S. who smoke.
In addition to the scientific evidence supporting the reduced exposure profile of the product, consumer understanding of the message also matters. The FDA found that our consumer perception studies show that consumers generally comprehend the modified risk information in the context of total health. In particular, the results indicate that consumers understand that the product is not without risks and that it is more harmful than quitting smoking. Consumers also generally perceive the product as less harmful than combusted cigarettes, which is in line with the relative health risks of the product that are reasonably likely.
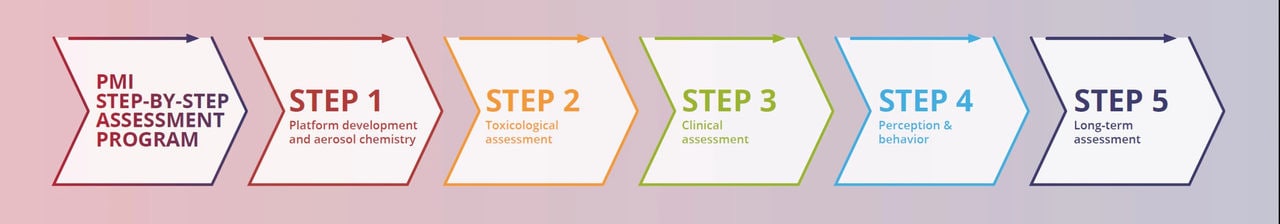
Our assessment program for the THS was presented to the FDA in our applications. This simplified, 5-step version represents the different levels of evidence we can obtain from our product assessment. Step 1 examines the product and its aerosol, Step 2 understands its effects using laboratory models, Step 3 learns about the effects in smokers who switch, Step 4 studies how people perceive and use the product, and Step 5 observes its impact on population health and/or over the long term.
Authorization: An ongoing process
The marketing order expires four years from the issue date of the FDA’s marketing order (by law, it can be no longer than five years). PMI can seek the renewal of the order by submitting a request to the FDA 360 days prior to the expiration date. The order also requires PMI to conduct postmarket surveillance and studies to determine the impact of these orders on consumer perception, behavior, and health, and to enable the FDA to review the accuracy of the determinations upon which the orders were based.
Importantly, youth should not use any tobacco or nicotine-containing product. While the FDA found that the currently available evidence suggests that youth uptake of THS is low in countries where it has been measured, given that youth are at increased risk, generally, for initiating tobacco use. Given the uncertainty around the effect of modified risk information on youth use, the FDA’s authorization includes postmarket requirements to help ensure that youth exposure to tobacco marketing is being minimized.
This includes informing the FDA of all advertising and marketing plans prior to dissemination, implementing plans to restrict youth access and limiting youth exposure to the products’ advertising. In addition, PMI is required to conduct postmarket surveillance and studies to monitor youth awareness and use of THS to ensure that marketing of the product as an MRTP will not have the unintended consequence of leading to increased use of these products among youth.
The FDA's decision to authorize the marketing of the IQOS Tobacco Heating System as a Modified-Risk Tobacco Product with reduced exposure Information demonstrates the fundamental difference between this product and cigarettes. The FDA has set a high standard, and we look forward to working with them to implement the order so that IQOS is reaching the right audience.
The FDA's decision to authorize the marketing of the IQOS Tobacco Heating System as a Modified-Risk Tobacco Product with Reduced Exposure Information demonstrates the fundamental difference between this product and cigarettes. The FDA has set a high standard, and we look forward to working with them to implement the order so that IQOS is reaching the right audience.
Final thoughts
The FDA’s decision to authorize the marketing of the IQOS Tobacco Heating System as a Modified Risk Tobacco Product with reduced exposure Information demonstrates the fundamental difference between the IQOS system and combusted cigarettes. The FDA has set a high standard, and we look forward to working with them to implement the orders so that the IQOS system is reaching the right audience. The marketing order allows adult consumers in the U.S. who would otherwise continue to smoke cigarettes to make a better and more informed choice.

Read the Scientific Update magazine
The Scientific Update magazine is focused on PMI's research and development efforts, milestone studies, industry regulations, and more. View the latest issue, or read the articles online.

