Tobacco harm reduction as a part of regulatory approach
Looking back at how governments have tackled the issue of smoking, the focus has primarily been on traditional prevention and cessation strategies. Although, the idea of regulating tobacco products originated in the 1960s with reports from the U.K. Royal College of Physicians and U.S. Surgeon General on Smoking and Health, regulatory policies have since then followed identifiable trends, including advertising restrictions, limiting the areas where one may use such products, health warnings, taxation, and more.
During the late 1990s, a group of global tobacco control experts was convened by the World Health Organization (WHO) and asked to explore how to strengthen efforts to reduce the harm caused by smoking. This group recommended that complementing prevention measures and cessation support programs with a harm reduction approach would significantly reduce smoking-related harm in future generations. A harm reduction approach can help adult smokers who would otherwise continue to smoke to have access to scientifically substantiated smoke-free products that are available today as a result of significant advances in technology and science.
More and more governments around the world have adopted the principles of harm reduction in their tobacco policies and have recognized that smoke-free products can have a role to play in reducing the harm caused by smoking. These governments are complementing traditional tobacco control measures such as those intended to discourage initiation and encourage cessation of smoking, with harm reduction approaches such as providing adult smokers that do not quit with information about, and access to, scientifically substantiated smoke-free products to accelerate the move away from cigarettes. Countries such as New Zealand and Czechia officially embraced a harm reduction approach as a complementary policy to address the issue of smoking and accelerate its decline. In the U.S., Italy, Bulgaria, Cyprus, Portugal, and Greece, regulators have adopted regulatory frameworks defining the scientific requirements for the communication towards the consumers about reduced-risk or reduced-harm effects of innovative tobacco products compared with smoking.
Categorizing smoke-free products
Many countries have updated their existing regulatory frameworks to regulate novel smoke-free products under unique or dedicated product categories. These categories include a level of differentiation compared with combustible tobacco products, including differentiated health warnings, flavor and packaging requirements, and others. This approach recognizes that not all tobacco products are equally harmful. Indeed, nicotine-containing products fall on a continuum of harm, with cigarettes at the highest end of the continuum.
Heated tobacco products (HTPs) have a significant potential to serve as an acceptable alternative for adults who would otherwise continue to smoke. HTPs, also known as heat-not-burn products, are a category of products that heat the tobacco instead of burning it. The aim is to substantially reduce the emission of harmful chemicals in HTPs compared as with cigarette smoke. This innovative and evolving category includes products that vary with respect to temperature, heating source, or the way the tobacco may be processed. While not risk free, HTPs can be a better alternative to smoking, and they should be made available to adult smokers who would otherwise continue to smoke.
Following a similar trend, e-vapor products are also classified as a unique product in many countries and regions. New Zealand or the EU—which has implemented the Tobacco Products Directive (TPD)—are examples of countries or regions which acknowledge that HTPs and e-vapor products are different from cigarettes.
However, there are also countries that take a more excessive approach and ban smoke-free products altogether, effectively allowing in the market only the most harmful form of consuming nicotine, i.e., cigarettes. Examples include India, Turkey, or Brazil.
On the other hand, there are also countries which have taken the approach of regulating smoke-free products the same as combustible products. This approach limits or outright bans the communication with adult smokers, which makes their informed decision making very difficult. These approaches disregard the potential role of scientifically substantiated smoke-free products can have in moving adult smokers who don’t quit away from cigarettes, the most harmful way of nicotine consumption.
Some countries take other unique approaches. In Australia, for example, nicotine is restricted and classified as a 'dangerous poison' by law. If it is used for therapeutic purposes, i.e., quitting smoking, nicotine products have to be registered under the Therapeutic Goods Act (1989). Therefore, with the exception of cigarettes, products containing nicotine are only recognized for use as smoking cessation therapy and require a prescription from a registered Australian medical practitioner. Regulatory mechanisms under this classification make it more difficult for adult smokers who don’t quit to access products whose risk profile is different from cigarettes.
Nicotine pouches, yet another smoke-free product category, don’t involve a device, heating, or the inhalation of an aerosol. Instead, these pouches are designed to be placed between the upper lip and gum, allowing nicotine absorption through oral mucous membranes before entering the bloodstream.
From a regulatory point of view, the approach to oral nicotine pouches varies. They are commercialized in the U.S., the U.K., and various countries in Europe. In the EU, the regulatory classification of nicotine pouches is not harmonized (i.e., not subject to EU directives) with some EU countries, explicitly or implicitly, banning the commercialization of nicotine pouches altogether (Belgium and the Netherlands), while others regulate them such as Czechia, Slovakia, Sweden, Denmark, Iceland, and Norway. Pending regulations are in progress in various countries across Europe such as Finland to more clearly integrate nicotine pouches within the tobacco product regulations.
An evolving regulatory landscape
New technologies continue to emerge, and regulations must continue to adapt at a rapid pace. In the heat-not-burn category, new products have been introduced which contain nicotine without tobacco, or which are based on other ingredients and do not contain either nicotine or tobacco. New production methods have even made it feasible for some companies to produce products with synthetic nicotine, or even which contain nicotine analogues like metatin, placing them outside the regulatory framework in some countries.
Priorities among health agencies and countries continue to change in response to these emerging products. The global discussion on tobacco regulation has seen a shift from a focus on tobacco and its health harms to zero in on nicotine instead. Nicotine is indeed addictive and not risk free, but it is not the primary cause of smoking-related disease. Refocusing regulatory attention to the primary cause of the health harms of smoking, which is combustion, and the ways these harms can be mitigated could have a positive impact on public health.
Another priority shift, or perhaps inconsistency, that has been seen is the promotion of public health policies that outright ignores tobacco harm reduction approaches. Consider the WHO Framework Convention on Tobacco Control (FCTC), where harm reduction is defined as a strategy of tobacco control. Despite this definition, harm reduction is absent from the recommendations of the WHO.
One important aspect of public health which several countries are already addressing is the importance of regulation that safeguards against access to tobacco and nicotine-containing products for underage users. These regulations will require the precision to balance these safeguards while ensuring that adult smokers who don’t quit are not prevented from accessing products that are better than continued smoking.
In the midst of this changing regulatory landscape, the primary role of regulation remains the same: protecting the health of the public. The reliance on data and scientific evidence to make informed policy decisions, and a harm reduction approach that sees the most harmful products being subject to the most restrictive regulations, have the potential to see the greatest impact on public health.
Looking ahead, policymakers and regulators will continue to update tobacco legislation in response to emerging tobacco and nicotine alternatives. Here are some examples of countries that are regulating smoke-free products or integrating the concept of tobacco harm reduction within their regulations.
Guided by government health assessment agencies’ conclusions that e-vapor products are significantly less harmful than smoking, the U.K. government has highlighted the role e-cigarettes could play in reducing the harms from smoking. In its 2017 5-year Tobacco Control Plan for England, it stated:
“[T]he evidence is increasingly clear that e-cigarettes are significantly less harmful to health than smoking tobacco. The government will seek to support consumers in stopping smoking and adopting the use of less harmful nicotine products.”
The government’s approach is reflected in the respective restrictions applicable on e-vapor products, which are subject to differentiated health warnings, greater opportunity to provide information to adult users, and lower taxes compared to cigarettes, supporting adult smokers to move away from smoking.
The U.K. government is also encouraging adult smokers to consider using e-vapor products through national initiatives such as the Stoptober campaign, a month-long national stop smoking challenge, and a ‘swap to stop’ scheme whereby one million smokers were encouraged to swap cigarettes for e-vapor products in a world-first national scheme. These measures, amongst others, aim to help the government meet its ambition of being smoke-free by 2030 (defined by the government as reducing smoking rates to 5% or less).
In 2018, the U.S. Food and Drug Administration (FDA) announced a new strategic plan to address smoking by increasing restrictions on cigarettes and by allowing “greater flexibility” for alternative non-combusted products. Through the FDA, the U.S. government allows the commercialization of new tobacco products after the product goes through authorization schemes for the introduction and marketing of such products into the U.S. market.
Under the Premarket Tobacco Product Application (PMTA) scheme, the FDA is responsible for assessing and authorizing the sale of new tobacco products, and determines, based on the assessment of the scientific evidence, whether new tobacco products are “…appropriate for the protection of the public health” with respect to the risks and benefits to the population as a whole, considering both users and nonusers.
In 2019, following the assessment of the PMTA for the tobacco heating system (THS), the FDA authorized the commercialization of our THS 2.2 in the U.S. In 2021, the FDA also authorized the first set of e-vapor products through the PMTA pathway.
In addition, under the Modified Risk Tobacco Product (MRTP) scheme, the FDA decides whether or not tobacco products may be marketed with modified health risk claims. The FDA issued, in 2019, an MRTP decision in respect of eight snus smokeless tobacco products marketed by Swedish Match USA—now a PMI company—under the General brand name. The FDA authorized the marketing of these products with the claim “Using General Snus instead of cigarettes puts you at a lower risk of mouth cancer, heart disease, lung cancer, stroke, emphysema, and chronic bronchitis.”
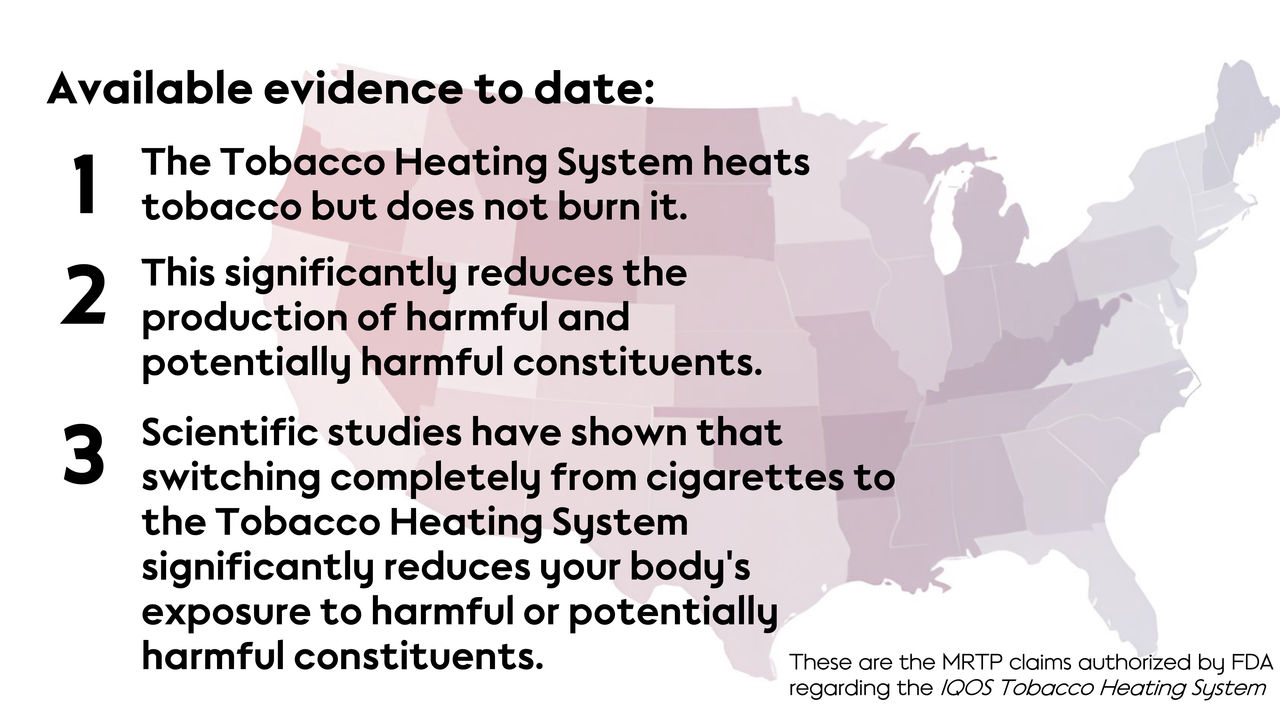
In 2022, the FDA authorized the MRTP for THS 2.2 with the following reduced exposure information:
- “The IQOS system heats tobacco but does not burn it.
- This significantly reduces the production of harmful and potentially harmful chemicals.
- Scientific studies have shown that switching completely from conventional cigarettes to the IQOS system significantly reduces your body’s exposure to harmful or potentially harmful chemicals.”
Also in 2022, Congress passed into federal law the regulation and enforcement of Non-Tobacco Nicotine (NTN) products, clarifying FDA’s authority to regulate tobacco products containing nicotine from any source, including synthetic nicotine. Similarly to tobacco products, such as cigarettes and smokeless tobacco, NTN products can only be legally marketed in the U.S. if they are authorized by the FDA through the PMTA pathway.
In 2018, the Ministry of Health recognized the role vaping products (legal definition of which includes e-cigarettes and HTPs) could play in achieving New Zealand’s smokefree target of 5% daily smoking prevalence by 2025, despite having previously banned them. Since then, the government has been vocal in encouraging adult smokers to consider vaping if they don’t quit altogether.
To support their 2025 targets, the government has implemented a risk-proportionate approach to the regulation of tobacco and nicotine products that clearly differentiates between smoked tobacco products on one side and smoke-free tobacco and e-vapor products on the other. The Smokefree Environments and Regulated Products (Vaping) Amendment Act 2020, which regulates e-vapor products and HTPs, has for purpose to “…support smokers to switch to significantly less harmful products.” The Act recognizes that these products are less harmful than combusted tobacco products and sets in place several concrete, risk-proportionate measures such as the following:
- The investment in public health communication campaigns to encourage switching and provide accurate information on vaping, including through a dedicated website called “Vaping Facts”.
- The removal of the requirement that “smokeless tobacco products” be sold in plain packaging.
- The adoption of different health warnings for e-vapor products and HTPs compared to cigarettes to allow more communication about “significantly less harmful products” for adult smokers.
- Retailers can display notices, instore or online, to help move adult smokers away from combustible products. Some of these include, “Completely replacing your cigarette with a vape will reduce harm to your health.” “If you smoke, switching completely to vaping is a much less harmful option.”
The Greek government has integrated harm reduction as a complementary approach within its tobacco control strategy. In 2019, the government presented the National Action Plan against Smoking that strengthens and supports the actual implementation of the anti-smoking law (Law 4633/2019), declaring harm reduction as the fourth pillar of its anti-smoking strategy along with prevention, elimination of second-hand smoking, and cessation support. This amendment allows communication of science-based claims for novel products.
This harm reduction approach was legislated in 2020 (Law 4715/2020) with the objective “…of achieving maximum harm reduction for active and passive smokers with the aid of new technologies, added to the policies of prevention at a young age, the cessation of smoking and the protection from passive smoking.”
The new legislation also recognizes the right of citizens to access information about special properties of certain tobacco products such as non-combusted tobacco products and the communication of reduced-risk messages subject to a strict procedure of scientific assessment led by a specialized committee.
In 2023, the Greek Ministry of Health approved differentiated health claims for HTPs, including THS.
Harm reduction is central to the approach taken to tackle smoking in the Czech Republic as indicated in the National Strategy to Prevent and Reduce the Harm Associated with Addictive Behaviour 2019-2027. The Action Plan for 2019-2021 included risk-proportionate product taxation and the use of smoke-free products to stop smoking:
- The Ministry of Finance was tasked to “Regularly increase excise duty on tobacco products while respecting the differentiation according to the degree of harmfulness of each product for the society.”
- The Ministry of Health and expert associations were tasked to “Prepare and approve the revision of the current recommendations for treatment of tobacco addiction in the area of harm reduction.”
- The Ministry of Health and Ministry of Agriculture were tasked “At the international level in the area of tobacco control to support the inclusion of the harm reduction principle where the measures are scientifically substantiated and do not undermine public health especially among kids, youths and non-smokers.”
This approach of science-based harm reduction was reiterated in the Action Plan for 2022-2025.
- The plan states: “[I]t is necessary to propose a legislative framework favouring less risky tobacco alternatives, nicotine and related products, and thus reduce the incidence of smoking cigarettes and tobacco products with burnt tobacco, and thus minimize the effects of smoking on population health in the Czech Republic.”
- It further states that the goal is “ ... to appropriately set the taxes (price) of alternative tobacco products and nicotine products in relation to the price of smoking tobacco products, and economically thus motivating smokers to switch to less risky tobacco alternatives.”
The Government Manifesto adopted in 2022 also reflected principles of risk-proportionate regulation:
- “Excise duties will take harmfulness into account.”
- “In addressing addictions, the Government will pursue a policy based on balanced and scientifically proven concept of risk prevention and harm reduction.”
- “The regulation of addictive substances will correspond to the degree of their harmfulness.”
In 2018, the Philippines House of Representatives adopted a resolution “…urging the Department of Health to promote harm reduction measures as part of its National Tobacco control strategy, particularly the use of electronic cigarettes as an alternative for smokers.”
The 2022 Vaporized Nicotine and Non-Nicotine Products Regulation Act No. 11900 was passed by overwhelming majorities in the House of Representatives and Senate. This Act reversed the ban on importing HTPs and adopts a state policy of tobacco harm reduction and risk-proportionate regulations by creating a separate regulatory framework for vaporized nicotine and non-nicotine products, such as e-vapor products, HTPs, and other novel tobacco and nicotine products. The regulation declares support for harm reduction as state policy by including “reduce the harm from smoking" in the Act’s Declaration of Policy.
The Act provides definitions for novel tobacco and nicotine products based on lack of combustion, allows communication about non-combusted alternatives targeted at adult smokers to facilitate switching, requires the registration of these products as novel consumer goods, sets technical standards for safety, consistency, and quality of these products based on international standards, and contains provisions on preventing youth access to these products.
Importantly, the regulations create a pathway for products that will be marketed with an explicit reduced risk statement to be authorized by the Philippine Food and Drug Administration.
In 2021, the Swiss Parliament adopted a new Tobacco Products Law regulating novel tobacco and nicotine products. The main features of the law included dedicated product categories for smoke-free products (such as e-vapor products and HTPs) and smokeless health warnings for products that do not combust, as well as allowing manufacturers to communicate factual, non-misleading science-based communications for non-combusted products on product packaging, except claims on curative, lenitive, or preventive effects, amongst others.
In the explanatory report to the Tobacco Law, the Swiss Government stated that “…the recognition of these alternative products and the elaboration of specific provisions can encourage smokers of conventional cigarettes to switch to a less harmful product.”
The Protection of Health (Tobacco Control) Law of 2017 in Cyprus provides rules for the “…approval for the use of accompanying indications according to which the use of a novel tobacco product presents reduced risk or harm for health compared to conventional tobacco products.”
The Portuguese Tobacco Law adopted in 2017 (and revised in 2023) recognizes harm reduction as a viable solution for adult smokers who do not quit: “Regardless of their legal nature, the health services, particularly health centres, hospitals, clinics, doctors’ surgeries and pharmacies, should promote and support health information and education for the citizens in respect of the ill effects of tobacco consumption and of the importance of prevention as well as of putting an end to tobacco consumption, through campaigns, programmes and initiatives directed at the population in general or at specific groups, particularly children and youths, pregnant women, parents, women of fertile age, the sick, teachers and other workers, and also, and exclusively to adult smokers that are not successful with cessation therapies, about the existence of alternatives that are acknowledged by the Directorate-General of Health, as of entailing harm and risk reduction.”
The Government Manifesto, adopted in 2023 by the Parliament, states support for harm reduction with respect to health policy.
“The Government will ensure the development, protection, and support of the public health of the entire society in Slovakia in the form of effective prevention and targeted programs. We will focus on prevention and harm reduction as a[n] interministerial topic and multidisciplinary issue, which is crucial from the point of view of improving the health status of citizens, as well as the sustainability of the health system. Part of the comprehensive approach of the care for the health of the citizens will also be the focus on the issue of the health determinants and addictions...”
“... This comprehensive approach will lead to a policy which will be data driven and applied will be a balanced combination of prevention and harm reduction of risk factors and behaviors.”
The concept of reducing the harm from smoking has been integrated in legislation enacted as far back as two decades ago. Tanzania’s primary tobacco legislation, The Tobacco Products (Regulation) Act, introduced in 2003, includes the concept of harm reduction to achieve its key objective “…to reduce tobacco use and its consequent harm by:
- protecting persons under eighteen and other non-smokers from inducements to use tobacco products;
- protecting non-smokers from exposure to tobacco smoke;
- ensuring that the population is adequately informed about the risk of using tobacco products and exposure to second hand tobacco smoke and about the benefits available for quitting smoking;
- ensuring that tobacco products are modified to reduce harm to such an extent as may be technologically and practically possible; and
- promoting a climate that will lead to a smoking-free atmosphere in all walks of life.”