Why regulations on smoke-free products matter
In the interest of tobacco harm reduction, adult smokers and nicotine users need accurate and nonmisleading information about smoke-free products. As we have learned, product information can help them to switch more completely. The U.S. is an example of a country where there are regulatory pathways that are required for the commercialization of and communication about novel smoke-free products, an approach that can help to ensure there is clear substantiation behind statements made across the product category. Despite some of the successes of this approach, the implementation can be complicated. Here, we provide examples from our own applications.
Dr. Matthew Holman
Vice President and Chief Scientific & Regulatory Strategy Officer in our U.S. offices
Matthew worked for more than 20 years with the U.S. Food and Drug Administration (FDA), most recently as Director of the Office of Science at the Center for Tobacco Products (CTP).
Key milestones and what they reveal
November 2015 was a major milestone within the tobacco industry. It saw the clearance by the FDA of the first tobacco products to be commercialized under the premarket tobacco product application (PMTA), a legal requirement for the introduction of any novel tobacco-containing product into the U.S. market. These products were eight snus smokeless tobacco products sold under the General brand name, by Swedish Match USA, Inc., now a subsidiary company of Philip Morris International (PMI). While snus was already available in the U.S. market through a number of companies, the eight General Snus products were the first to go through this formal review. Submitted on March 11, 2015, the PMTA took 236 days to be authorized by the FDA, well within expected timeline.
Milestones in U.S. Tobacco Regulation for General Snus.
With the PMTA review completed, focus then turned to the Modified Risk Tobacco Product (MRTP) review for General Snus products, the application for which had been submitted in June 2014. An MRTP authorization allows the specified product to be marketed with reduced-risk information. The MRTP review by the FDA took 1,961 days to complete, and in October 2019, the use of the following claim was authorized: “Using General Snus instead of cigarettes puts you at a lower risk of mouth cancer, heart disease, lung cancer, stroke, emphysema, and chronic bronchitis.”
MRTP authorizations are valid for up to 5 years. As the MRTP authorization for General Snus products expired in October 2024, a renewal application was submitted in July 2023 by Swedish Match. In November 2023, the FDA completed a preliminary review of this application and determined that it met the filing requirements for a tobacco product seeking a modified risk order. As a result, the application was filed and entered the substantive review phase. The FDA opened a docket in early December 2023 and invited public comments on the renewal of existing MRTP orders. In June 2024, the MRTP application was referred to the Tobacco Products Scientific Advisory Committee (TPSAC), a group of experts in medicine, medical ethics, science, or technology involving the manufacture, evaluation, or use of tobacco products. Finally, in November 2024, the FDA issued a renewal of the modified risk granted orders for the eight General Snus products, allowing continued use of the reduced-risk claim when marketing these products. This is the first renewal of modified risk granted orders issued by FDA.
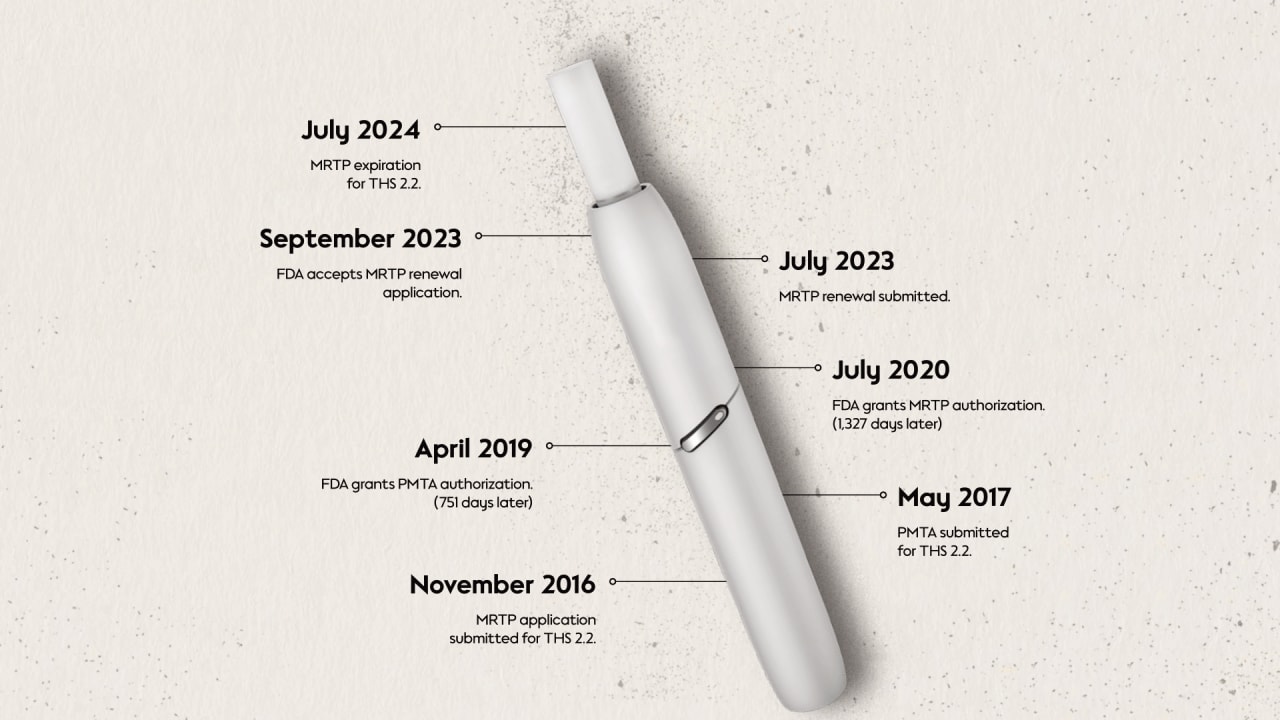
In May 2017, PMI submitted the PMTA for the tobacco heating system, Tobacco Heating System (THS) 2.2, commercially known as IQOS Originals. The FDA granted its authorization—the first heated tobacco product to be authorized under the PMTA in April 2019. The review of the MRTP application for the same product followed. Submitted in 2016, the MRTP review was completed in July 2020, and PMI was authorized to use the following statements:
- “The IQOS system heats tobacco but does not burn it.
- This significantly reduces the production of harmful and potentially harmful chemicals.
- Scientific studies have shown that switching completely from conventional cigarettes to the IQOS system significantly reduces your body’s exposure to harmful or potentially harmful chemicals.”
Milestones in U.S. Tobacco Regulation for THS 2.2.
This MRTP authorization has a 4-year expiration date, which ended in July 2024, and an MRTP renewal was submitted by PMI in July 2023. The FDA accepted this renewal application in September 2023 but has not provided any further response at the time of writing.
In January 2025, the FDA authorized the marketing of the PMTA pathway. This milestone marks the first time the agency has authorized oral nicotine pouches in the U.S. market. After a rigorous scientific review, the FDA determined that these products meet the regulatory standard. Among the key considerations in the FDA’s evaluation was that these products contain substantially lower amounts of harmful constituents than cigarettes and most smokeless tobacco products, which may translate into a lower risk of cancer and other serious health conditions. Evidence also showed that a substantial portion of adults who use cigarettes and/or other smokeless tobacco products completely switched to these oral nicotine pouches, but that youth use remained low despite growing sales.
An increasingly unpredictable market
One key challenge in the U.S. regulatory environment for tobacco products is the unpredictability in the time it takes the FDA to review marketing applications. For example, the PMTA for the 20 oral nicotine pouches commercialized as ZYN was submitted in March 2020, but authorization was not granted until January 2025—almost 5 years later.
PMTA authorizations have been granted by the FDA for various smoke-free products so far, yet a considerable number of PMTAs are still pending review at the FDA, with PMI applications among them.
In October 2023, PMI submitted PMTAs and MRTP applications for a new variant of THS, THS 3.0 commercialized as IQOS Iluma, and the accompanying tobacco sticks commercialized as TEREA. At the time of writing, the FDA has not yet confirmed the acceptance of these applications.
As the industry continues to push forwards with innovative smoke-free products, it is becoming increasingly difficult to predict when these products will be allowed to be commercialized in the U.S. market.
Unclear evaluating standards
Another challenge in the U.S. is the standards by which the FDA grants authorizations under the PMTA and MRTP pathways. While the FDA makes its assessments based on the risks and benefits of a tobacco product to the population, including both users and nonusers of tobacco products, the requirements for these assessments are available in draft format only and subject to change, for the MRTP process for example, leaving the opportunity for shifting priorities.
Because of the current ambiguities around the assessment process, PMI has sought out regulatory experts on the scientific evaluation of tobacco product applications at the FDA to guide us through these applications, and even then, the process remains difficult.
As the industry continues to evolve and brings forth more innovative products, the regulatory landscape must adapt to support its growth. Through PMTAs and MRTP applications, the regulatory approach taken by the U.S. has already embraced the harm reduction approach to complement prevention and cessation strategies linked to smoking cigarettes. Such an approach allows more adult smokers to choose lower risk options instead of continuing to smoke, which can have a positive impact on population harm. Reducing authorization timelines and clarifying standard requirements would be important for this approach to truly take hold in the U.S. and make a significant impact on public health.