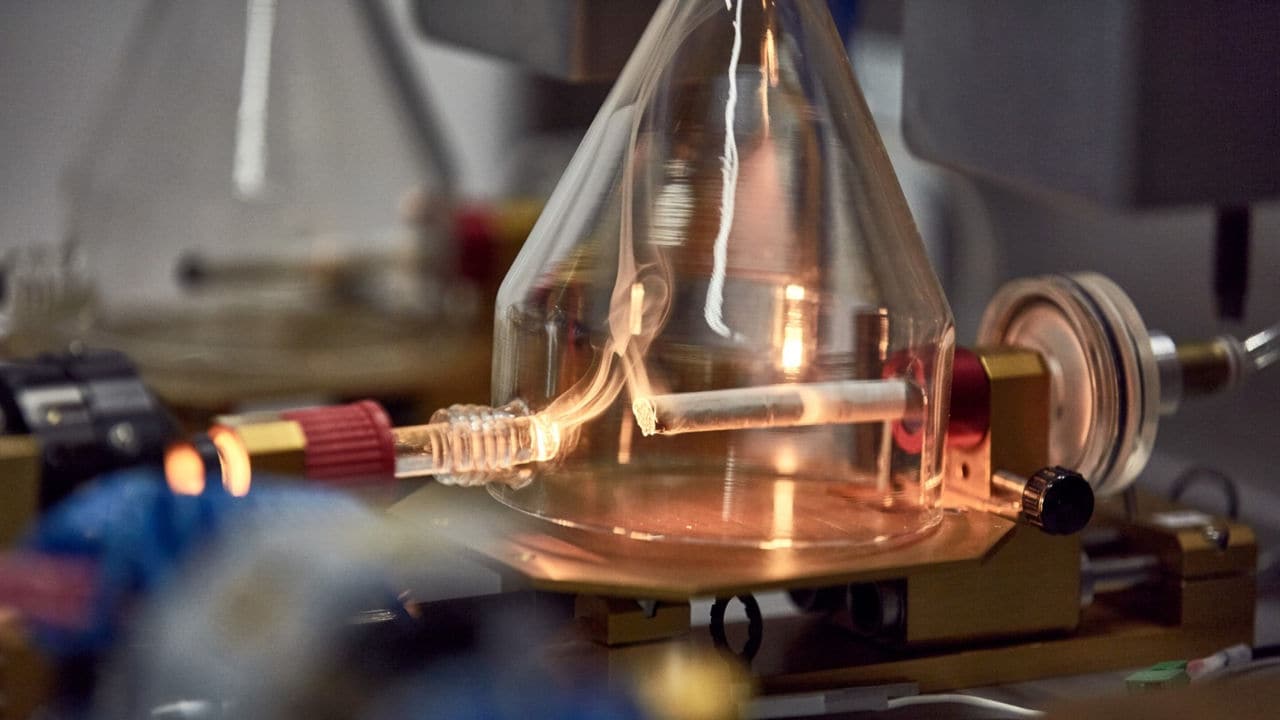
Why we don't need combustion
First: what is combustion?
Combustion is the process of burning something, and it requires three elements: a fuel source, like tobacco; oxygen, found in air; and enough heat to kickstart the self-sustaining, heat-generating reaction. During combustion, in ideal conditions, hydrocarbons and oxygen react to form carbon dioxide and water. In more realistic settings, for example in combusted cigarettes, there is not enough oxygen available for complete combustion to occur. Incomplete combustion leads to the production of carbon monoxide and numerous other molecules, of which many have been recognized by health authorities to be harmful or potentially harmful. Some of the toxicants emitted during combustion form liquid and solid particles that, together with the rest of the emissions, result in smoke that can be harmful to health if inhaled.

The best choice: stop smoking completely
Smoking a cigarette involves combustion and the generation of smoke. Millions of smokers every year decide that they don’t need cigarettes in their lives and quit completely. They make the single best possible choice a smoker can make for their health: they quit tobacco and nicotine completely. Still, there are many men and women who decide that while they don’t want to smoke, they do want to continue using nicotine and tobacco products. They are looking for similar taste, rituals, and nicotine uptake as those of cigarettes, but without the smoke. Thanks to technological advancements in smoke-free products, those experiences can be delivered without combustion.
We need heat—not combustion—to release nicotine
Nicotine in cigarette smoke is transferred from the tobacco at temperatures up to its boiling point around 247°C. That’s far below the temperature at which tobacco begins to burn, around 400°C.1 That means it’s possible to heat tobacco enough to release nicotine without burning it and producing smoke. HTPs do exactly this, for example. Alternatively, e-vapor products heat an e-liquid to release nicotine and flavors, while also avoiding combustion and smoke.

Tobacco—like many materials—loses mass into the air as it is heated through a range of temperatures.1 First, the drying process: it loses moisture between 30-150°C. From around 100-300°C, various chemicals besides water are evaporated from the tobacco as a result of the heat alone, and some of the tobacco components are broken down. Nicotine is also released from the tobacco at these temperatures.
Above this range, the presence of oxygen begins to become important as the tobacco draws closer to the ignition temperature. Below about 400°C, there’s not too much of a difference between the processes that occur in tobacco that’s heated in air with oxygen versus tobacco that’s heated without oxygen. But above 400°C, there’s a big difference: in oxygen, it burns.
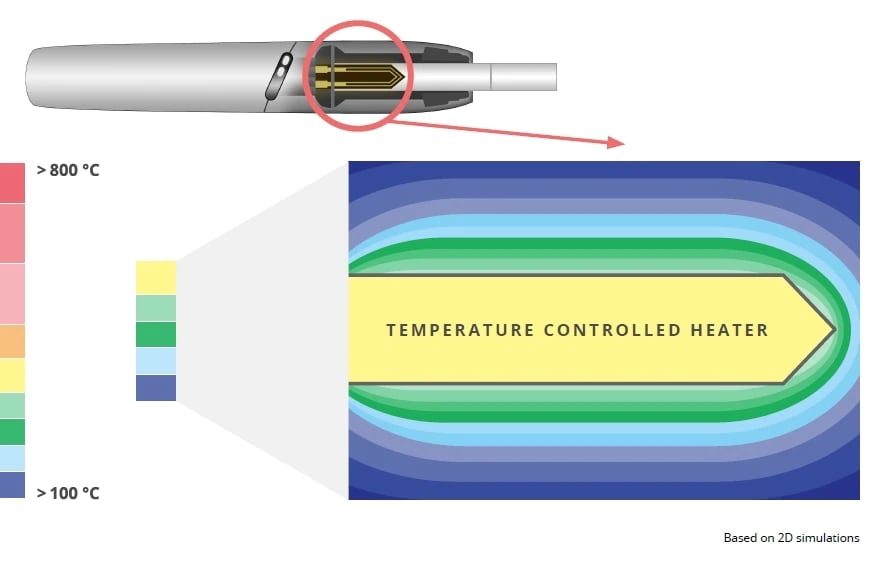
In our Tobacco Heating System (THS), there’s no combustion.2 The temperature of the tobacco is carefully controlled. It simply never reaches a high enough temperature, and the composition of the aerosol further verifies this. THS operation does involve heating, evaporation, torrefaction (think: roasting coffee beans), and some low-temperature pyrolysis, but no combustion. Further, the processes that occur in the THS do not create smoke, which is why we call it a smoke-free product.
Temperature of tobacco material in THS compared with cigarettes
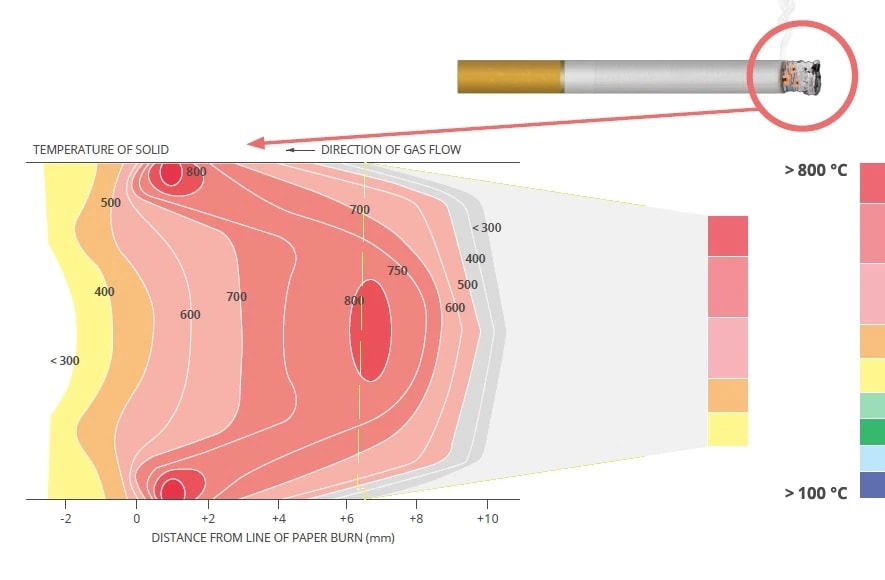
The temperatures of burning tobacco in a cigarette range from 600°C to over 800°C in the hottest regions, while the tobacco touching the heating blade in EHTS doesn’t rise high enough to burn the tobacco.
Chemicals in tobacco smoke
Thousands of components have been identified in tobacco plants, and it’s estimated that thousands more are waiting to be discovered, according to Rodgman and Perfetti.3 Like any plant, tobacco takes simple elements and converts them into macromolecules for growth and self-maintenance. That process is further compounded by the burning of its cured and dried leaves to create the complex mixture of more than 6000 chemicals that constitute tobacco smoke. Here are a few components of that smoke as described by Rodgman and Perfetti, 2013.
Cigarette smoke contains certain metals because tobacco plants require essential minerals for growth, and because the metals are also found in soil. Almost every metal found in tobacco can transfer in small amounts into cigarette smoke when the tobacco is combusted. Among these, arsenic, beryllium, chromium, nickel, and cadmium are listed as human carcinogens by the International Agency for Research on Cancer (IARC) as of 1985.
N-Nitrosamines are organic compounds recognized as carcinogenic, they’re formed during tobacco processing and during the burning of a cigarette. N-Nitrosamines may be categorized as tobacco-specific N-nitrosamines (TSNAs), as volatile or non-volatile N-nitrosamines (VNAs or NVNAs). The transfer of these constituents to smoke varies per N-nitrosamine. For example 6.9-11% of the TSNA N-nitrosonornicotine (NNN) is reported to be transferred from tobacco plant to smoke, and so is ~40% of the TSNA nicotine-derived nitrosamine ketone (NNK).
There are over 1200 hydrocarbons in tobacco smoke, including alkanes, alkenes, alkynes, alicyclic hydrocarbons, monocyclic aromatic hydrocarbons, and polycyclic aromatic hydrocarbons (PAHs). Over 500 tobacco smoke PAHs are known, many of which have been extensively studied because of their link to potential health concerns. This has led to the classification of PAHs as toxicants.
Knowledge about tobacco smoke constituents grew vastly in the 1950s, and as a result a list of specific aldehydes (such as formaldehyde, acetaldehyde, acrolein) and ketones (such as 3-pentanone; 4-heptanone; and 2,3-butanedione) were identified, which were reported to play a role in tumor formation and in ciliastasis—stopping the beating of mucous membrane cilia.
These are just a few of the thousands of chemicals and constituents that comprise tobacco smoke, many of which have been identified as harmful and potentially harmful constituents (HPHCs). Research has shown that a majority of HPHCs in cigarette smoke are caused by combustion. By eliminating combustion, we can reduce the level of HPHCs produced. Our studies have also shown an average reduction in THS aerosol HPHCs of 95%, compared with cigarette smoke.

Read the Scientific Update magazine
The Scientific Update magazine is focused on PMI's research and development efforts, milestone studies, industry regulations, and more. View the latest issue, or read the articles online.