A decade of safety data supporting the Tobacco Heating System
What data does PMI collect on its smoke-free products?
PMI collects safety data related to adverse events (AEs). These are health-related events that are not necessarily caused by product use but are associated with it. Data collected include information on the type of event (thermal burn, headache, cough, etc.), type of device, and the location, gender, and age of the reporter. As part of the medical review process, AEs collected from various sources are categorized by level of seriousness based on International Council for Harmonization (ICH) and European Medicines Agency (EMA) criteria.
According to these criteria, a serious AE is one which could result in one or more of the following:
- Inpatient hospitalization or prolongation of existing hospitalization
- Persistent or significant disability/incapacity
- Congenital anomaly/birth defects
- Is life threatening
- Death
- Is assessed as an important medical event
What do the data show about the overall safety of THS?
Our postmarket safety surveillance data show that, between 2014 and the end of 2024, THS has shown a steady safety profile, even as the number of markets where it is available expanded from two to more than 80 and the number of users increased from 5,000 (2014) to 32.2 million (end of 2024).
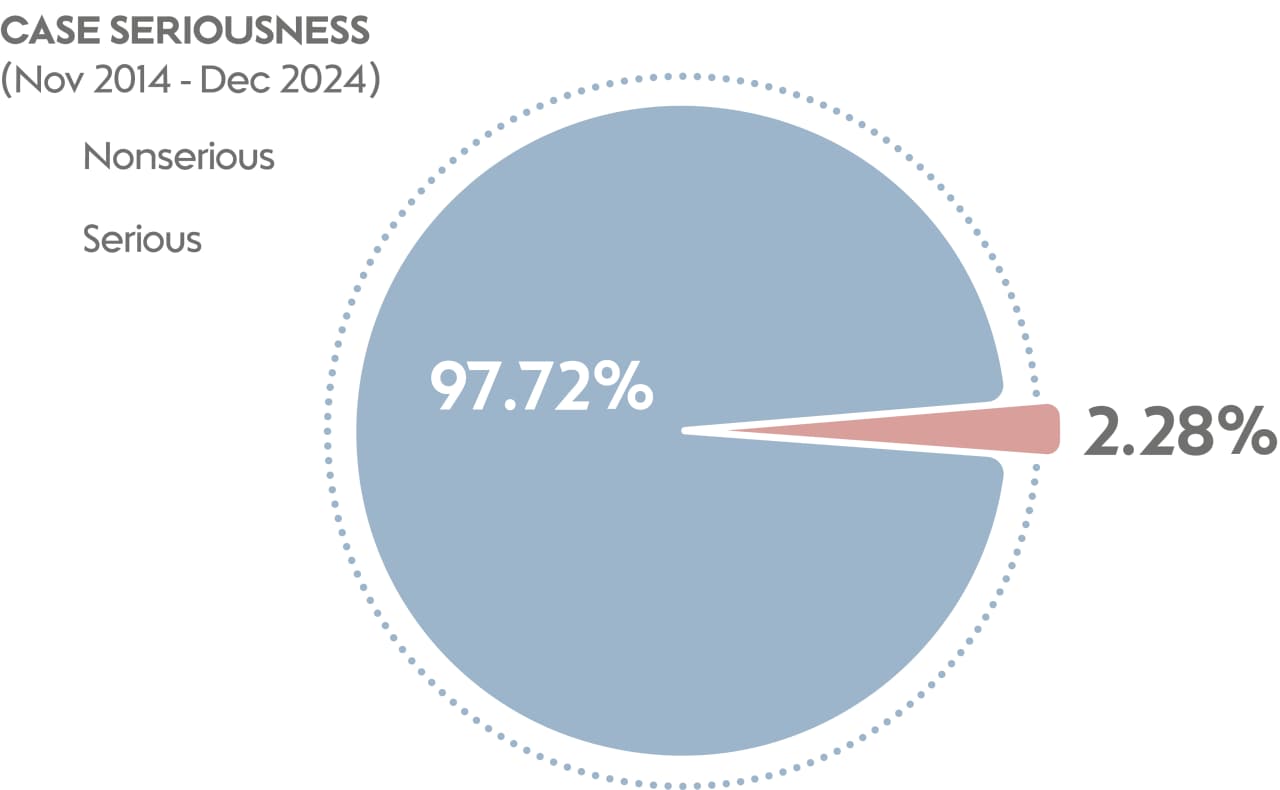
Throughout the past 10 years, the total number of AEs has been influenced by factors such as the number of markets, number of users, and events such as COVID-19. However, the percentage of AEs meeting the criteria for seriousness has remained stable at an average of 2.28% of the total number of AEs recorded. This is close to the safety profile for nicotine replacement therapies (NRTs), which serves as a reference for evaluating the safety of THS products. It is important to note that the number of serious events has also remained consistent across different devices, indicating that all PMI smoke-free products share a similar safety profile.
What are the most commonly reported AEs?
The overall data show that, for legal age consumers, the most frequently reported AEs for THS are similar to those most frequently reported with NRTs (the specific risks and frequency of reporting vary based on the type of NRT).
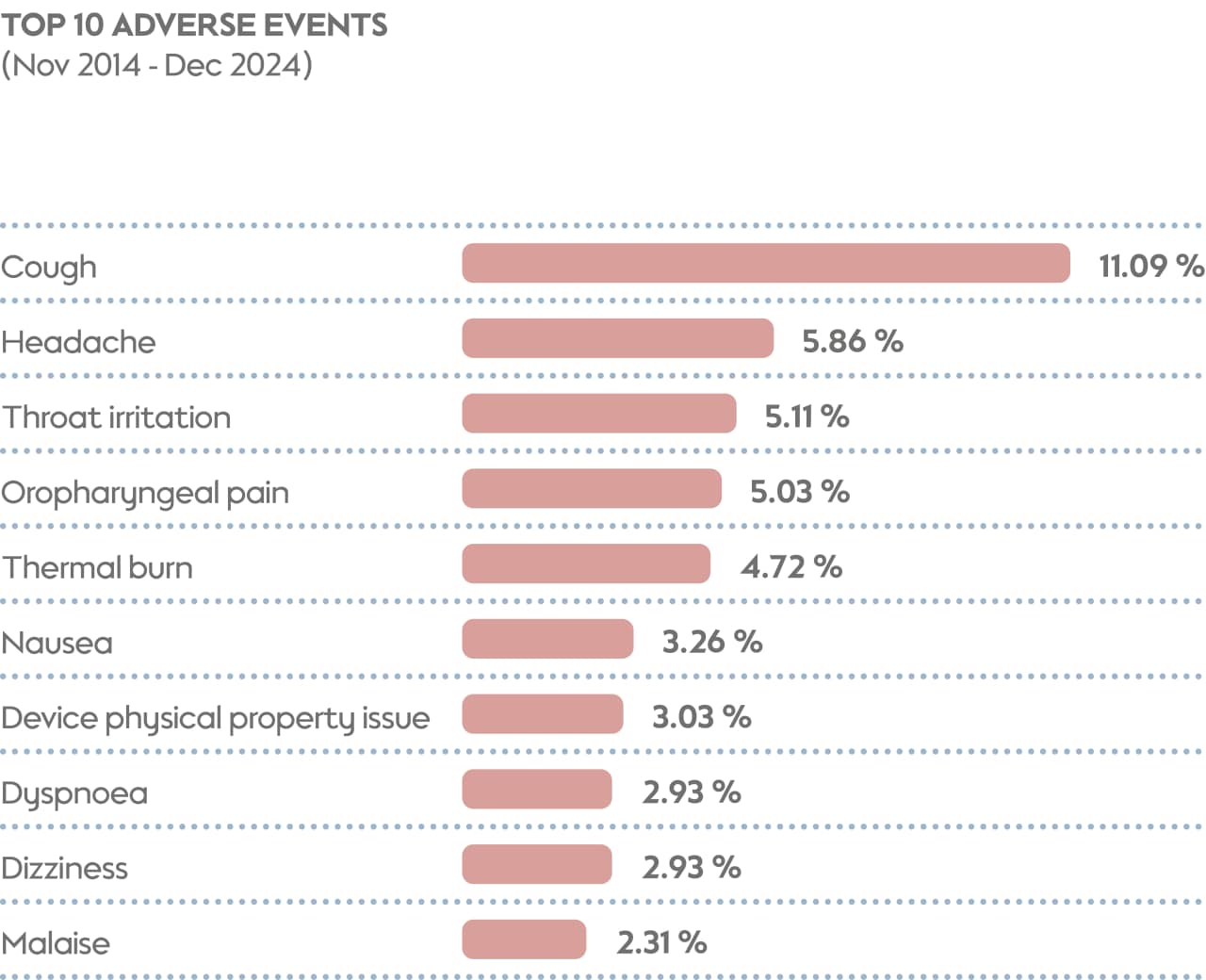
Cough is the most commonly reported AE for THS, representing just over 11% of AEs. Analysis of our postmarket safety surveillance data indicates that coughing tends to be most common in the first few days after switching from cigarette smoking to THS use. This might be related to the adaptation phase associated with the product. In addition to cough, headache, throat irritation, and oropharyngeal pain each account for more than 5% of reported AEs.
How do reported AEs for THS vary across countries?
As of the end of 2024, THS was available in more than 80 markets. We would expect to see a variation in the number of AEs reported in different markets. For example, we would expect countries with larger numbers of THS users to have more reports of AEs. In general, this is indeed the case.
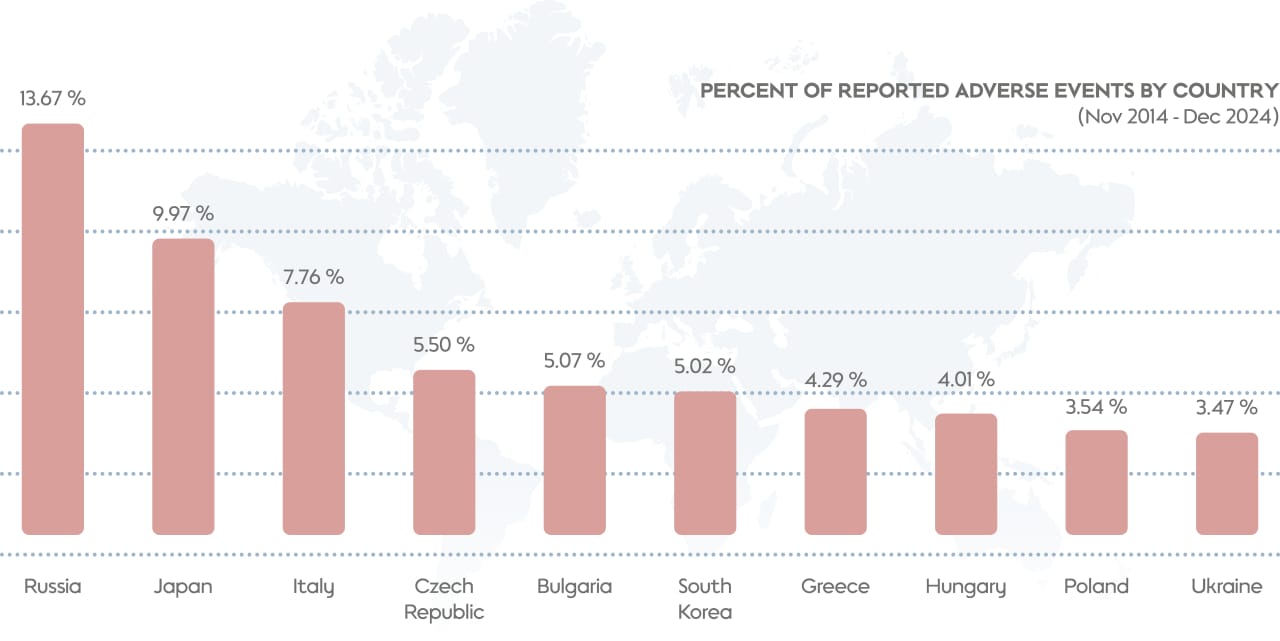
Another factor, however, is how familiar people in different regions are with the product. For example, THS was launched in Japan in 2014, so people in that country are familiar with the product and with the most common AEs (such as cough when initially switching to THS use from cigarette smoking) and, as a consequence, these events tend to be reported less frequently relative to the large number of users in the country. In contrast, users in new markets tend to report AEs more frequently, as they are less familiar with the recently introduced smoke-free product.
Climate conditions can also have an impact. For example, reports of thermal burn are more common in hot climates, and at times of year with hotter weather. However, looking at the top AEs across different regions, the safety profile for THS shows similar patterns globally.
How is postmarket safety surveillance data used for regulatory purposes?
Our postmarket safety surveillance program is used to support regulatory submissions and authorizations of our smoke-free products, such as the U.S. Food and Drug Administration's (FDA's) premarket tobacco product applications (PMTAs) and modified risk tobacco product (MRTP) applications. The information we collect also helps us meet reporting requirements needed to maintain our products on the market.
Systematic analyses of aggregated reports are conducted to uncover emerging trends and potential risks, facilitating the timely identification of safety signals. PMI scientists compile safety update reports based on these analyses, and this information is communicated annually to regulatory authorities.
In the European market, postmarket safety surveillance is a critical component of the EU General Product Safety Regulation 2023/988 (EU GPSR). This regulation ensures that products sold within the EU, both online and offline, meet high safety standards to protect consumers. It requires manufacturers to establish a comprehensive safety surveillance system that includes monitoring product performance, product accident reporting to authorities by businesses, appropriate management of products found to be unsafe, and continuous communication with authorities for transparency and effective management of risks.
AE cases that satisfy criteria for reporting under this regime are escalated internally to the product safety surveillance team who coordinates the review of the package and collects all the different documents to perform the submission.
THS is not a medical device, but by committing to the same rigorous processes as those used for medical devices, PMI is demonstrating that product safety surveillance is a core part of our commitment to harm reduction.
How does PMI track incidents related to accidental ingestion?
The safety surveillance team monitors reports of accidental ingestion involving PMI smoke-free products and also uses data from poison control centers to look at general patterns for whole product categories, for example, for all nicotine pouches on the market. The data is analyzed to assess how reports of accidental ingestion evolve over time and how this compares with the internally tracked reporting patterns for PMI products.
While there have been cases reported of accidental ingestion of PMI’s nicotine pouches, snus, e-vapor liquids, and THS consumables, no fatal or life-threatening acute nicotine toxicity cases have been reported so far.
Overall, the risk of lethal or life-threatening acute nicotine toxicity from accidental exposure to tobacco sticks, nicotine pouches, or portion-packed snus is considered highly unlikely. Our internal estimates indicate that, using conservative estimates for nicotine content and percentage of nicotine extraction, it would require more than 75 tobacco sticks or 40 nicotine pouches to be accidentally ingested at one time in order to produce serious or life-threatening effects in a 70 kg adult.
What is the overall safety profile of THS?
The data PMI collects on postmarket safety surveillance demonstrates that our THS has maintained a steady safety profile, even as the number of users and markets sees a large increase. These data cover 10 years of safety surveillance, from the launch of THS in 2014 until the end of 2024. Importantly, 97.7% of cases are nonserious, with the most common AEs reported (≥5%) being cough, headache, throat irritation, and oropharyngeal pain.