OECD 28-day and 90-day studies and the assessment of Reduced-Risk Products
The Organisation for Economic Co-operation and Development (OECD) guidelines for the testing of chemicals are a collection of the most relevant internationally agreed upon testing methods for assessing, identifying and characterizing potential hazards of new and existing chemical substances. In particular, testing guidelines 412 and 413 have been designed to fully characterize the toxicity by the inhalation route for a limited duration (28 or 90 days respectively) and to provide robust data for inhalation risk assessments[1][2].
OECD 412 and 413 to assess Reduced-Risk Products
We use OECD 412 and 412 to demonstrate:
that there is no increased toxicity of Reduced-Risk Product (RRP) aerosols compared to cigarette smoke, allowing the initiation of consumer-based clinical trials of defined duration, and
to quantify, by use of the various OECD-defined endpoints, if there is a reduction in toxicity of RRP aerosols compared to cigarette smoke.
OECD+
We usually supplement our OECD studies with additional investigations in order to better understand the mechanistic changes that occur on exposing rodents to aerosols using systems biology approaches. This allows for the determination of the total biological impact of RRP aerosols compared to cigarette smoke.
Rodents in OECD 28-day and 90-day studies
We use outbred male and female Sprague-Dawley rats bred under specified pathogen-free conditions in our studies. Upon arrival, their health status is verified by histopathological examination and serological screening. We always ensure that body weights are within 20% of the mean weight for each gender and the animals are 6-10 weeks old at the start of the study.
Example of a 28-day inhalation study
A 28-day inhalation study has been carried out, investigating all toxicity endpoints required by OECD 412 and supplemented with systems toxicology aspects such as transcriptomics.
All parameters specified in the OECD 412 were assayed with a focus on local effects in the respiratory tract. To provide an estimate of the smoke / aerosol concentration and dose inhaled, the steady-state blood carboxyhaemoglobin (COHb) concentration, respiratory physiology parameters and the concentration of representative urine nicotine metabolites are determined.
In order to characterize the biological activity of the smoke/aerosol, hematological, clinical-chemical, gross pathological and histopathological analysis of the major organs are measured. In addition, pulmonary inflammation markers are also measured.
We demonstrated reduced biological activity of the mainstream aerosol from a prototypic RRP (pRRP) compared with the mainstream smoke from 3R4F. Rats were exposed to filtered air, to three concentrations of mainstream smoke from 3R4F (8, 15 or 23 μg nicotine/l), or to a pRRP aerosol targeted to match the concentration in the high 3R4F group (23 μg nicotine/l). Histopathological analysis of respiratory tract tissues revealed concentration-dependent changes in response to 3R4F that were irritative-related in nasal and bronchial epithelium and inflammation-related in the lung parenchyma. For the pRRP, significant changes relative to air exposure were seen in the nasal epithelium only.
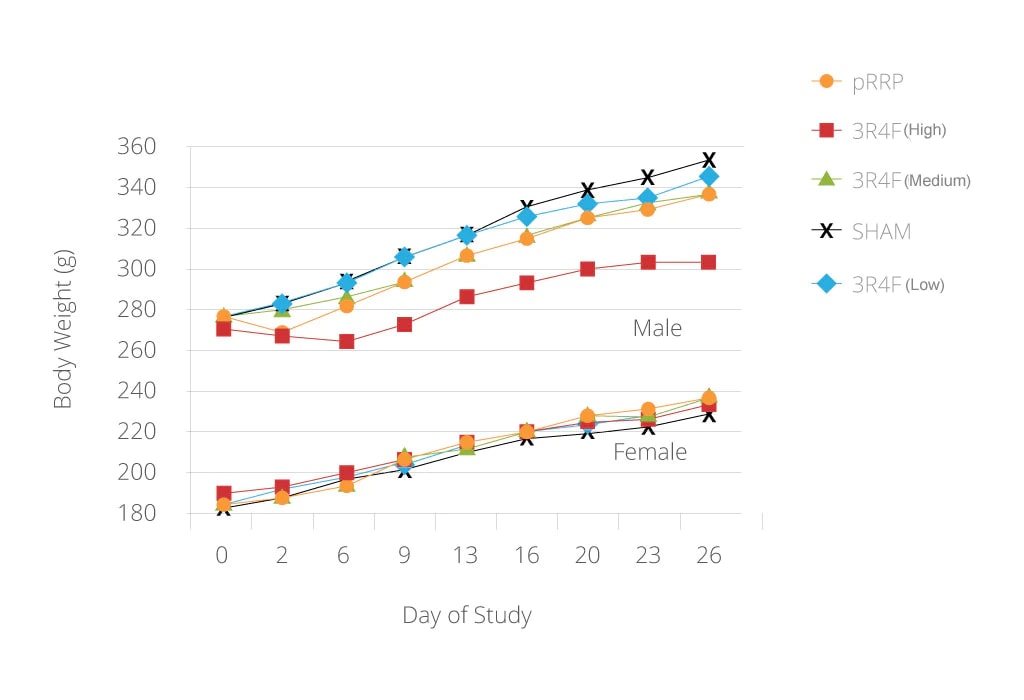
Mean body weight development of male and female rats
Note: these data alone do not imply or represent a claim of reduced exposure or reduced risk.
As shown in the diagram, body weight development revealed an exposure level-related reduction for 3R4F(high)-exposed male rats. The absence of a mainstream smoke-related decrease in body weight gain in female rats is consistent with results from previous studies. Note the absence of exposure-related body weight changes in the males rats exposed to the pRRP.
Male and female rats of the cigarette mainstream smoke-exposed groups showed a concentration-dependent increase in absolute numbers of neutrophils, macrophages, lymphocytes and eosinophils in their lungs (investigated in broncho-alveolar lavage fluid). The number of inflammatory cells in the pRRP-exposed rats was not different compared to air-exposed rats and much lower than in the rats exposed to mainstream smoke from cigarettes.
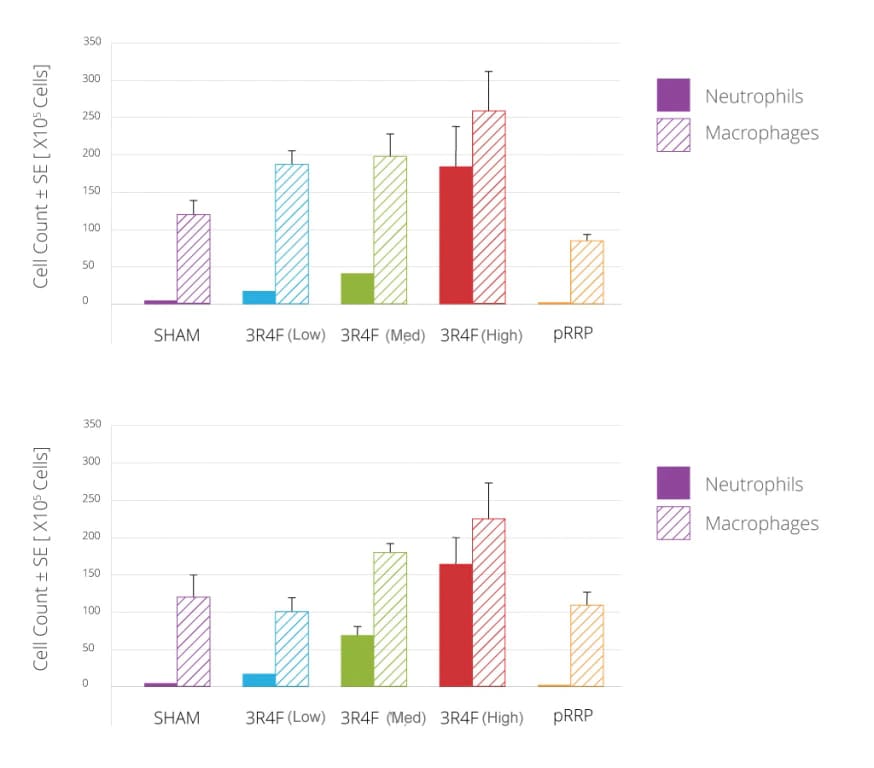
Macrophages and neutrophils in broncho-alveolar lavage fluid.
(A) Male rats, (B) female rats. Values are absolute numbers per lung (means ± standard error).
Note: these data alone do not imply or represent a claim of reduced exposure or reduced risk.
As an example of histopathological effects in the respiratory tract of the rats, significant mainstream smoke exposure-related effects, eg, reserve cell hyperplasia and squamous metaplasia with and without cornification, were seen in the respiratory epithelium (nose levels 1-2) as well as atrophy in the olfactory epithelium (nose levels 2-4) for both male and female rats of the 3R4F group.
For the pRRP, mainstream smoke exposure-related effects were only observed in the respiratory epithelium (nose levels 1-2) and were consistently lower than those in the 3R4F(high) group, or showed no significant mainstream smoke exposure-related effects.
Histopathological findings from nose level 1, respiratory epithelium.
The amount of cornification was scored. Values are mean scores ± standard error. Solid bars are female rats, patterned bars are male rats.
Note: these data alone do not imply or represent a claim of reduced exposure or reduced risk.
References:
[1] OECD. OECD guideline for the testing of chemicals: subacute inhalation toxicity 28-day study. 2009. Available online at: http://www.oecd-ilibrary.org/environment/test-no-412-subacute-inhalation…
. [2] OECD. OECD guideline for the testing of chemicals: subchronic inhalation toxicology 90-day study. 2009. Available online at: http://www.oecd-ilibrary.org/environment/test-no-413-subchronic-inhalati…