The Mouse Lymphoma Assay and assessment of Reduced-Risk Products
The Mouse Lymphoma Assay (MLA) is a widely used in vitro genotoxicity assay that can detect gene mutations induced by different types of exposures. It uses the L5178Y mouse lymphoma TK+/- cell line, detecting mutagenic events by measuring resistance to the pyridine analogue triflurothymidine (TFT).
The MLA to assess Reduced-Risk Products
The MLA has been shown to be effective as part of the comprehensive toxicological assessment of cigarette smoke and Reduced-Risk Product aerosols[1] and we employ the assay using a methodology underpinned by the Organisation for Economic Co-operation and Development (OECD) guidelines 476[2].
To date we have used the MLA to test an early prototype version of our Reduced-Risk Product Platform 1 called the Electrically Heated Cigarette Smoking System Series K (EHCSS). The results are shown in the graph below where a significant reduction in mutations can be seen between EHCSS and 2R4F[3].
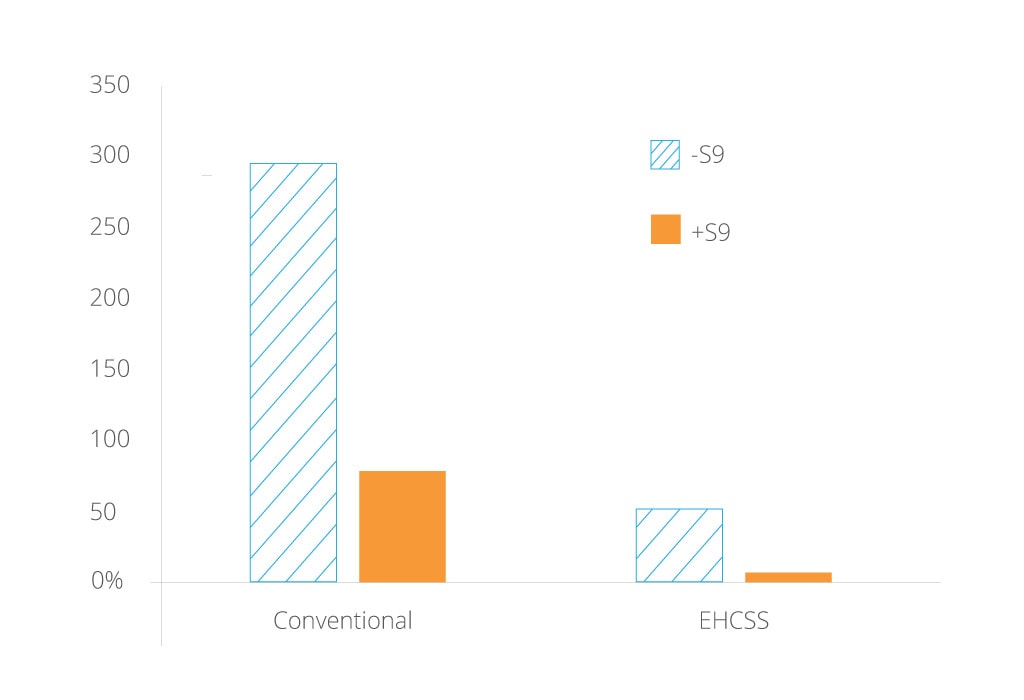
Reduction in mutations between EHCSS and 2R4F. S9 fraction describes a range of metabolic enzymes derived from organ tissue. Chemical substances sometimes require metabolic activation in order to become mutagenic. Addition of a S9 tissue fraction provides the enzymes required for such activation. Note: these data alone do not imply or represent a claim of reduced exposure or reduced risk.
References:
[1] Schramke, H, et al. The mouse lymphoma thymidine kinase assay for the assessment and comparison of the mutagenic activity of cigarette mainstream smoke particulate phase. Toxicology, 2006. 227(3): p. 193-210. Available online at: http://www.sciencedirect.com/science/article/pii/S0300483X06004689
[2] OECD. OECD guideline for the testing of chemicals: in vitro mammalian cell gene mutation test. 1997. [3] Werley, MS, et al. Smoke chemistry, in vitro and in vivo toxicology evaluations of the electrically heated cigarette smoking system series K. Regul Toxicol Pharmacol, 2008. 52(2): p. 122-39. Available online at: https://www.sciencedirect.com/science/article/abs/pii/S0273230008001177