Brindusa Taranu
Head Product Safety Surveillance

Brindusa Taranu
Head Product Safety Surveillance
Provides oversight for the revision and optimization of processes related to safety monitoring, safety signal detection, and documentation of safety cases. Oversees all critical activities related to high-profile safety concerns, emerging safety issues, and litigation-relevant safety topics. Serves as the most senior safety point of contact for other departments during the issue escalation process.

Nicolas Blanc
Principal Medical Safety Officer
Provides medical interpretation of case-level information and performs medical risk assessments, starting with the design and control principles of the products and extending throughout the commercialization phase.

Nikolina Nuic
Manager Safety Databases
Oversees the safety surveillance platforms to ensure optimal performance, compliance with data integrity guidelines, and effective system vendor management.

Kamila Kowa
Senior Safety Lead
Analyzes the data in the safety database to identify trends and potential new safety signals, performs signal detection management, and prepares safety reports that are submitted to health authorities.
Brindusa: Postmarket safety surveillance is the practice of monitoring the safety of a product after it has been released on the market. In order to improve consumer safety, we conduct systematic collection, analysis, and communication of health-related events—known as adverse events (AEs). These are events that are not necessarily caused by product use but are associated with it. PMI launched its safety surveillance system alongside the initial release of the Tobacco Heating System (THS), in 2014. We currently conduct postmarket safety surveillance for all our smoke-free products, which include heated tobacco products, e-vapor products (aka e-cigarettes or vapes), and nicotine pouches.
This system is used to ensure timely detection and regulatory-compliant handling of safety signals to protect consumer well-being, support responsible innovation, and contribute to product improvement.
Kamila: In 2019, the U.S. Food and Drug Administration (FDA) authorized the marketing of the THS 2.2 under the premarket tobacco product application (PMTA) pathway. Under the PMTA, it is a legal requirement that any novel, tobacco-containing product introduced into the U.S. market must be supported by scientific data demonstrating that the product is appropriate for the protection of public health. We are also obliged to collect all AEs that occur with the usage of our smoke-free products and perform signal detection activities. In our annual report to the FDA, we include an update and analysis of any safety signals and risks that were identified for our smoke-free products. So, our safety surveillance system ensures we meet these requirements.
Brindusa: Data from our smoke-free products are collected from more than 80 markets, and some of these markets have their own reporting requirements. Greece, for example, requires the reporting of all serious AEs, while Canada requires only reporting of life-threatening AEs and deaths. In addition to the U.S., our safety data help us meet reporting requirements in around 35 countries where our products are marketed.
Nikolina: To track health-related events, we use a database called LifeSphere® MultiVigilance (LSMV), which is a common tool used in the pharmaceutical industry for safety surveillance. There are many ways that information on AEs can come to us, but around 90% of all AEs come through PMI’s call centers. What often happens is that a consumer calls to ask a question, maybe about how to use the product, and during the call they mention an AE, for example, headache after product use. The call center agents are trained to recognize AEs, so when they hear that someone has experienced a health-related event, they open LSMV and fill out the special form that we have created for them.
Brindusa: LSMV is a validated system that incorporates advanced technology and follows pharmaceutical industry standards inspired by the EU Good Pharmacovigilance Practices (GVP). We continuously evaluate and enhance the system with new tools and technology to improve efficiency. For example, we recently integrated the LifeSphere® Signal and Risk Management (LSSRM) tool. This is an end-to-end platform for the definition, identification, management, and communication of safety risks throughout the lifecycle of a product. This platform enhances our ability to rapidly identify signals and respond appropriately, further supporting consumer protection.
Nikolina: Artificial Intelligence (AI) is used to extract specific information about events from the AE reports. The LSMV system also uses intelligent coding, which automatically assigns AEs to a standardized classification system and applies criteria for determining their seriousness and expectedness. Approximately 80% of our cases are processed automatically.
Brindusa: The Identification of discrepancies or missing information is also partially automated: the system will suggest questions for the call center to ask, and the team will verify whether those questions are relevant to a particular query and issue the final instructions to the call center.
Kamila: We perform both quantitative and qualitative analysis on the data we receive. In our qualitative review we focus on specific cases that meet one or more of the seriousness criteria, and we conduct a medical review to determine if our product was causally related to a serious AE. We also analyze cases quantitatively, in aggregate, to look for patterns. For example, if we notice a spike in the numbers of a specific AE, we conduct a deeper quantitative analysis to try and understand what has caused the observed increase. We then use statistical analysis to verify if that increase was statistically significant.
Nicolas: We also examine the data for signals. A signal is an event which represents either a potential or an identified risk whose occurrence is likely enough to justify further analysis and verification. Signals may include an unexpected event that might be linked to a product but for which we lack confirmed evidence, or a previously identified risk where there is enough evidence to show a link with the product.

Nicolas: When we have identified a signal, we conduct a risk assessment, taking into account the severity of the situation and the likelihood of it occurring. This will produce a score that tells us the seriousness of this risk.
Kamila: The level of seriousness will affect the action taken. For example, it will help us determine if placing a warning on the product is adequate to mitigate the risk, or if the risk is sufficiently severe and likely to occur that we need to consider a withdrawal of the product from the market. Generally, we put standard mitigation measures in place to limit the risk, no matter the severity. Even if it is something relatively minor, we want to make sure our consumers are aware of it.
Nicolas: There are two main ways in which our work informs product development. For products that are already on the market, we notify the product development teams when a new signal is identified. If it is a serious risk, they will investigate the feasibility of making changes at the product level. In addition to this, we will change the safety warnings to inform consumers of the risk. For products not yet on the market, we can use the experience we gained from conducting safety surveillance on existing products to advise product development teams during the design phase.
In 2016, safety surveillance data indicated an increase in cases of thermal burns being reported to PMI’s call centers. Complaints mainly included burns to the lips, fingers, or hands while using THS.
The safety surveillance team reviewed and analyzed the reports and determined that thermal burn was in fact a safety signal related to product use. Further analysis determined that the physical perception of thermal burn was driven by an increase in aerosol mass, which occurred when tobacco rods absorbed more moisture after being stored in humid conditions. The increase in aerosol mass can raise the temperature by 3 °C or more, causing the aerosol to reach temperatures hot enough to be perceived as mildly-to-moderately painful. The team concluded that most of the cases of thermal burn were reports of this burning sensation rather than tissue damage.
Quantitative examination of the AE reports found that thermal burn complaints tended to spike during periods of hotter and more humid weather. These spikes were due to the consumables absorbing more water, increasing the aerosol mass and the subsequent number of thermal burn reports.
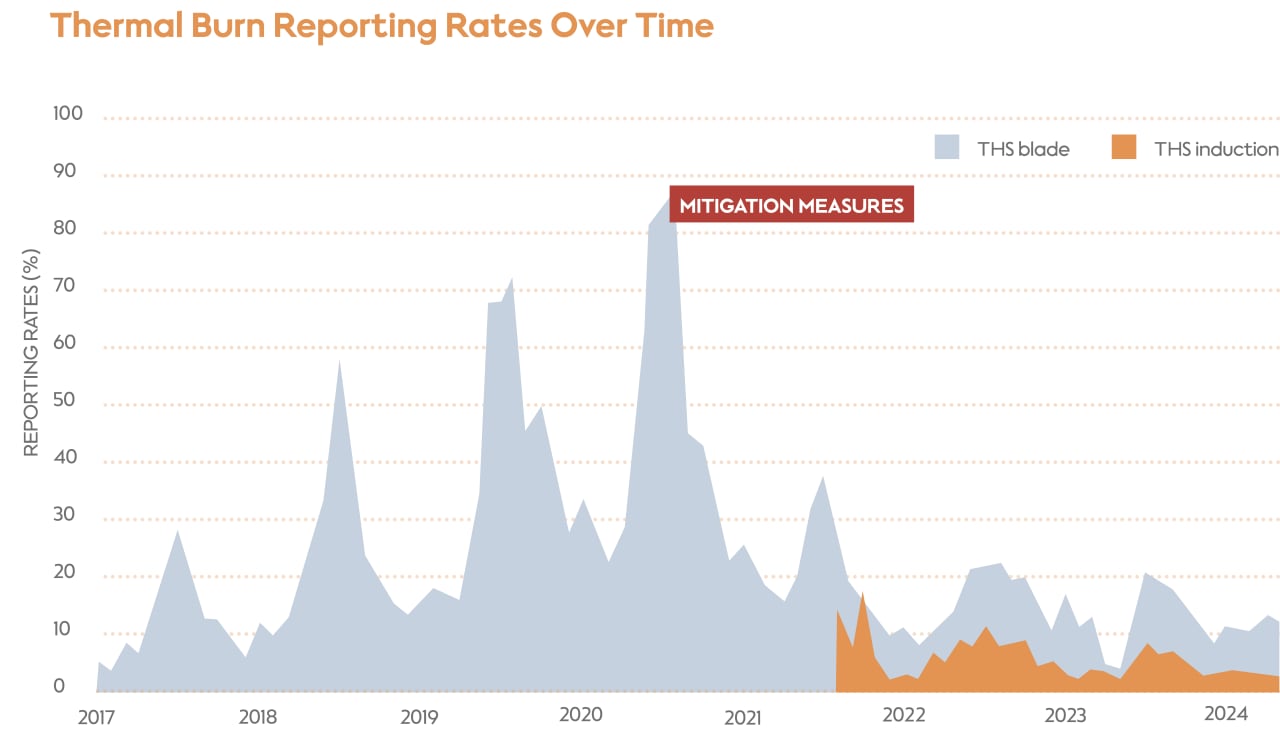
Reporting rates were calculated by dividing the number of cases by the number of estimated consumers in millions.
Once the cause of the signal was determined, actions were taken to warn consumers and mitigate the risk. This included redesigning the consumables to increase airflow, subsequently decreasing the temperature of the aerosol.
Consumer safety warnings and instructions in the user guide were updated to highlight the specific risk and advise users to keep consumables in a cool, dry place. An analysis of the thermal burn risk was also included in annual reports submitted to relevant authorities, and ongoing monitoring of thermal burn reports continues through PMI’s safety surveillance system.

Brindusa: PMI participates in a number of initiatives through CORESTA (Cooperation Center for Scientific Research Relative to Tobacco), an international organization promoting global scientific cooperation in tobacco research. As the tobacco industry has no current guidelines for conducting postmarket safety surveillance, PMI, together with other tobacco manufacturers, are working to put this in place under the CORESTA umbrella.
Through CORESTA, PMI is working to address the lack of standardized guidelines for postmarket surveillance of smoke-free products. The initiative, which was proposed by PMI and approved in April 2024, will develop best practices and guidelines inspired by pharmaceutical pharmacovigilance systems.
This effort aims to harmonize AE management across the tobacco industry, support regulatory compliance, and enhance product safety through a phased approach that includes guideline development and industry-wide training. The proposal aligns with both CORESTA’s mission and our own Product Science team’s 5-year plan, and includes key industry stakeholders.
Nikolina: While all tobacco companies are conducting safety surveillance, the level at which we do it is significantly more advanced. That’s why PMI’s participation in CORESTA is important, it’s an opportunity to work together with other tobacco companies to improve safety surveillance across the industry.
