Evaluating the toxicology of electronic nicotine delivery products
“Toxicological Evaluation of Electronic Nicotine Delivery Products”, a new book by PMI Scientists including Dr. Manuel Peitsch, Chief Scientific Officer, and Dr. Julia Hoeng, Global Head Discovery, examines the potential role of Electronic Nicotine Delivery Products (ENDPs) in tobacco harm reduction and how they may reduce the risk of smoking-related disease in smokers who switch to them.

“The orange color of the book cover was selected as a reminder that ENDPs, while less harmful than cigarettes, are also not risk-free.” - Prof. Manuel Peitsch, Chief Scientific Officer
This latest publication provides toxicologists, health practitioners and public health professionals with the scientific information necessary to assess the relative risk of ENDPs compared with continued cigarette smoking and smoking cessation, which clearly remains the best choice.
A substantial body of epidemiology has demonstrated that smoking cigarettes increases the risks of serious diseases, such as lung cancer, heart disease, and chronic obstructive pulmonary disease (COPD). The best way to avoid the harm from smoking is to never start, or for current smokers to quit tobacco and nicotine use altogether.
The purpose of tobacco harm reduction is to accelerate the reduction in risk of illness and death associated with smoking tobacco. It does so by combining measures for reducing smoking prevalence, especially those that deter initiation and encourage stopping, with measures that reduce exposure to toxicants in smokers who would otherwise continue smoking.
This book explains that developing new products that are scientifically substantiated to present less risk of harm compared to cigarettes is one approach. ENDPs are one category of alternative products that use electronics to control an electrical system and heat either tobacco, as in heated tobacco products (HTPs), or a nicotine-containing liquid, such as e-vapor products. For an ENDP to qualify as a less harmful alternative to cigarettes, it must be scientifically proven to emit and expose users to significantly lower levels of toxicants, and to cause significantly less adverse effects than cigarettes.
Scientifically substantiated ENDPs that avoid combustion of tobacco have been shown to emit significantly lower levels of toxicants than cigarettes. The extent of that reduction should however be scientifically assessed for each product. They also generate aerosols that are respirable but may dissipate more quickly than cigarette smoke. In addition, the reduction in toxicant emissions by ENDPs also leads to a reduced impact on indoor air quality relative to cigarettes.
“This book shows that the scientific findings across many studies and scientific disciplines are coherent along the causal chain of events linking smoking to disease and consistent across studies. It also shows that both heated tobacco products and e-vapor products have the potential to reduce harm and the risk of disease.”
- Prof. Manuel Peitsch, Chief Scientific Officer
What’s in cigarette smoke?
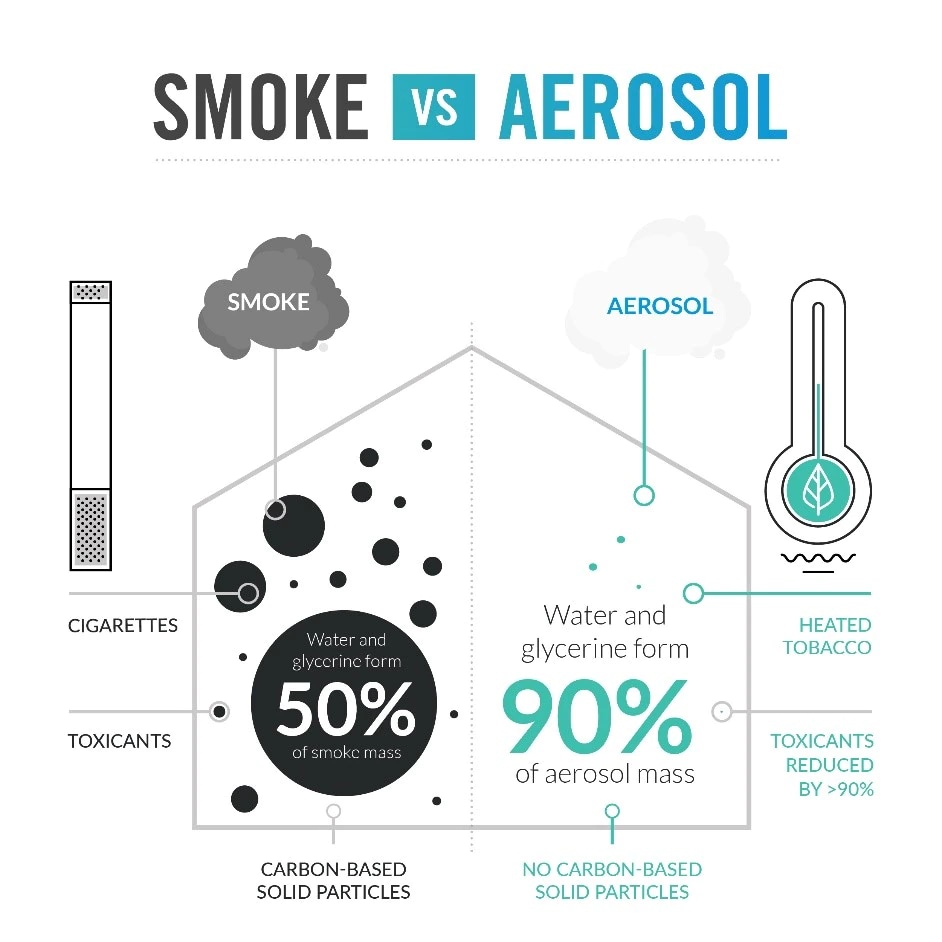
The smoke produced from a lit cigarette is a mixture of solid and liquid particles that contains more than 6,000 constituents. Among these chemicals, public health authorities and others have identified around 100 harmful and potentially harmful constituents (HPHCs) that are linked to smoking-related diseases.
The heat-not-burn principle
HTPs are different from cigarettes because they do not rely on the principle of burning. They are designed to heat tobacco to a precise temperature without burning it, to produce a nicotine-containing aerosol with significantly lower levels of HPHCs than cigarette smoke.
The authors argue that the potential public health benefits of ENDPs depend on their potential to reduce the risk of smoking-related disease and their acceptability as alternatives to cigarettes by adult smokers. In addition, ENDPs should not attract unintended users such as former smokers, people who have never smoked, and youth.
Heated tobacco products and their aerosols
The book describes studies showing that the aerosols generated by the ENDPs under investigation are less toxic than cigarette smoke in vitro and in vivo, across a wide range of biological mechanisms and endpoints at the cellular, tissue and physiological levels.
The evidence available to date does not indicate that switching from cigarette smoking to scientifically substantiated ENDPs leads to new identifiable health risks, as they are designed to heat tobacco or an e-liquid and generate inhalable aerosols that are far less complex.
“The book summarizes key concepts and results from tobacco harm reduction research over the past 15 years. Even though it focuses on research done by PMI, it also includes key findings from other researchers. As such, it has the potential to be a reference document for anyone wanting to learn how to best assess such products and see the scientific evidence backing their promise for a smoke-free future.”
- Dr. Stephanie Boue, Manager Scientific Transparency and Verification
The book highlights the importance of distinguishing between the risks attached to nicotine use, which is addictive and not risk free, and the risks posed by other chemicals in cigarette smoke. Disassociating the health effects strictly attributable to nicotine from those presented by the other chemicals typically generated by nicotine-containing products is challenging.
There are still gaps in the scientific knowledge of the biological effects of nicotine. The currently available evidence on the effects of nicotine and cigarette smoke shows that “most of the harm caused by smoking arises not from nicotine but from other components of tobacco smoke” and that “nicotine is not a highly hazardous substance”.
For smokers, however, quitting tobacco and nicotine altogether is the best option, and nicotine-containing products should not be used during pregnancy, breastfeeding, and development, or by people who have or are at risk of heart disease.
Harm reduction policies
The evidence available for those ENDPs that went through scientific assessment shows that they emit reduced levels of toxicants. However, these products are not risk free and provide nicotine, which is addictive. For this reason, ENDPs are only intended for current adult smokers or users who would otherwise continue to use tobacco or nicotine-containing products.
For the harm reduction approach to be successful, manufacturers must be able to provide truthful and nonmisleading information to consumers, including that the best choice they can make is to quit tobacco or nicotine use altogether, and for those who won’t, that there are acceptable alternatives to continued smoking. Regulation should include strict rules for scientific substantiation and vigorous enforcement of consumer protection laws. Policymakers should also aim to provide the public with accurate and balanced information.
Current adult smokers must have access to accurate and nonmisleading information about ENDPs toxicity and risk of harm relative to continued cigarette smoking. This is crucial for encouraging them to switch completely from smoking cigarettes to using ENDPs, if they would otherwise continue to smoke.
“Toxicological Evaluation of Electronic Nicotine Delivery Products”, which is published by Academic Press (14th January 2021), may be ordered via the following link:


