PMI’s advanced research: smoke-free future and beyond
Investing in scientific expertise
Our work combines cutting-edge research with innovative technology to further our understanding of how our smoke-free products impact consumer health. This has generated a wealth of scientific data, spanning everything from aerosol characterization and toxicological analyses to clinical studies that compare the effect of smoke-free product use with that of cigarettes on exposure to harmful chemicals and the risk of smoking-related diseases. Additionally, perception and behavior research and long-term studies provide insights into the contribution of smoke-free products to population health and use patterns. Our scientists have also built advanced computing systems to store and analyze the immense volume of data generated by our research.
Whilst the primary purpose of the Cube is to advance PMI’s smoke-free product research and evaluation, our efforts extend beyond this, allowing us to contribute more broadly to the fields of research in which we specialize.
Here are a few examples of the research areas PMI is currently exploring and how these efforts could influence research beyond smoke-free products.
Exploring how the human airway interacts with inhalable agents
Given that aerosols from smoke-free products are inhaled into the lungs, it is no surprise that our scientists are experts in understanding the interactions between aerosols and the human airway—the path through which air reaches the lungs. This research is undoubtedly essential for advancing our knowledge of the science behind our smoke-free products, but it can also offer valuable insights for scientists working on inhalable therapeutics.
Everyone’s airway is unique. Factors like a person’s age, gender, weight, fitness, and disease status all affect the shape and functioning of their airway. Comprehending how differences in the human airway impact its interaction with inhaled agents is important for studying the adverse effects of inhaled substances, uncovering how respiratory diseases might develop, and improving the delivery of therapeutics. To this end, PMI scientists have been working alongside other research teams to develop accurate, more reliable models of the human airway.
For example, our researchers used 3D organotypic cell cultures—generated from samples from different donors—that closely resemble real human lung tissue to investigate how different donor characteristics affect lung shape and function. The scientists demonstrated that 3D cell cultures were able to function and metabolize in a similar way, despite varying from donor to donor in shape and response to stimuli.
PMI researchers were also part of a team that created a model of the human airway that can be personalized to reflect an individual’s unique airway. This model can be used for person-specific simulations of breathing, gas exchange, and aerosol deposition. Information from these two models can be extended to therapeutics, helping scientists better understand how inhaled pharmaceuticals are delivered to the body, evaluate their efficacy, determine or predict adverse effects before they occur, and personalize treatments based on physiological differences that can influence treatment outcomes.
Building on this work, PMI scientists developed the independent holistic air-liquid exposure system (InHALES), an aerosol exposure device that closely resembles the human airway in both structure and function. It captures the effects of medical inhalers or the aerosols from tobacco and nicotine-containing products on different parts of the human airway in a single experiment, providing invaluable dosing information for inhalable agents and aiding in the development of therapeutics.
Systems toxicology to predict health impacts
Evaluating potential toxicity linked to smoke-free products is a crucial part of our research at PMI. Systems toxicology investigates how external stimuli affect entire biological systems or organs. At PMI, we use systems toxicology to compare the effects of using smoke-free products or cigarettes at various biological levels—molecular, cellular, tissue, organ, and whole organism—to identify disruptions that might lead to disease. This approach and our methods can be applied more broadly to help evaluate the risks of chemicals, drugs, and consumer products.
Predicting how a new compound will impact the human body—both its intended and nonintended effects—is a core challenge in biomedicine. By combining complex network models and standard endpoints from preclinical and clinical studies with technologies that rapidly aggregate vast data, systems toxicology can help evaluate the effects of a drug or chemical on an entire biological system.
The effects of drugs and chemicals on a biological system can be measured through biomarkers. These are natural molecules or signals found in your body that help scientists detect diseases, monitor disease progression, or measure response to treatments or exposure to chemicals or toxins.
At PMI, much of our research looks at biomarkers of potential harm (BoPH), which measure an effect, like that caused by exposure to a chemical. This can be a change in physiological shape or function, or clinical symptoms that signal an increased risk of disease. We use BoPH to assess the impact of switching from cigarettes to smoke-free products on the risk of developing diseases like chronic obstructive pulmonary disease (COPD), cardiovascular disease (CVD), or lung cancer.
In 2024, PMI scientists completed a large study investigating the effect of switching from cigarettes to smoke-free products on BoPH. This cross-sectional study collected real-world data from 982 participants across 37 healthcare institutions in 6 countries. Compared with current smokers, switching to smoke-free products for at least 2 years was associated with favorable differences in BoPH related to biological pathways that are negatively impacted by cigarette smoke. These data build on previous findings from our 6-month exposure response study and its 6-month extension and helps us better understand and scientifically substantiate the reduced-risk potential of our Tobacco Heating System (THS) in real-world conditions.
Lipidomics in toxicological research
Lipidomics, the study of cellular lipids, is crucial for understanding the biological impacts of smoke-free products. Integral to cell membranes, lipids perform a variety of vital functions in our bodies. Changes in lipid metabolism are reported to be linked to the development of several diseases.
At PMI, our lipidomics research focuses on identifying biomarkers associated with exposure to tobacco and nicotine-containing products and their possible health impacts. This is particularly important as many available biomarkers are not specific enough for accurate assessment of the risk of developing certain diseases.
By integrating lipidomics into our research, we generate advanced data-driven scientific evidence of harm reduction and contribute to the discovery of new biomarkers for evaluating the long-term effects of smoke-free products. To this end, we have adopted a high throughput shotgun lipid analysis method, alongside conventional techniques. Shotgun lipid analysis allows simultaneous detection and quantification of hundreds of molecular lipid species in various tissue and biofluid samples or in vivo systems. This adaptable, comprehensive analytical method could provide insights into the mechanisms of disease and toxicology and help identify biomarkers of early toxicity or beneficial effects in response to external stimuli.
Reducing animal studies in research
The use of animal models presents several ethical concerns and these models do not always accurately translate to human biology. Where possible, PMI aims to move away from dependence on the use of animals in research by adopting the 3Rs principle: reduce the number of animals used, refine procedures to minimize suffering, and replace animal models with alternative methods. To do this, we have adopted innovative methods, like organ-on-a-chip devices and adverse outcome pathways (AOPs).
Organ-on-a-chip devices offer a promising alternative to animal models because they can mimic human organs and organ systems in vitro. This technology places 3D cell cultures in an environment that simulates that of a human organ. By placing different “organs” on a single chip, we can gain insights on how these organs or organ systems interact and influence each other’s biology.
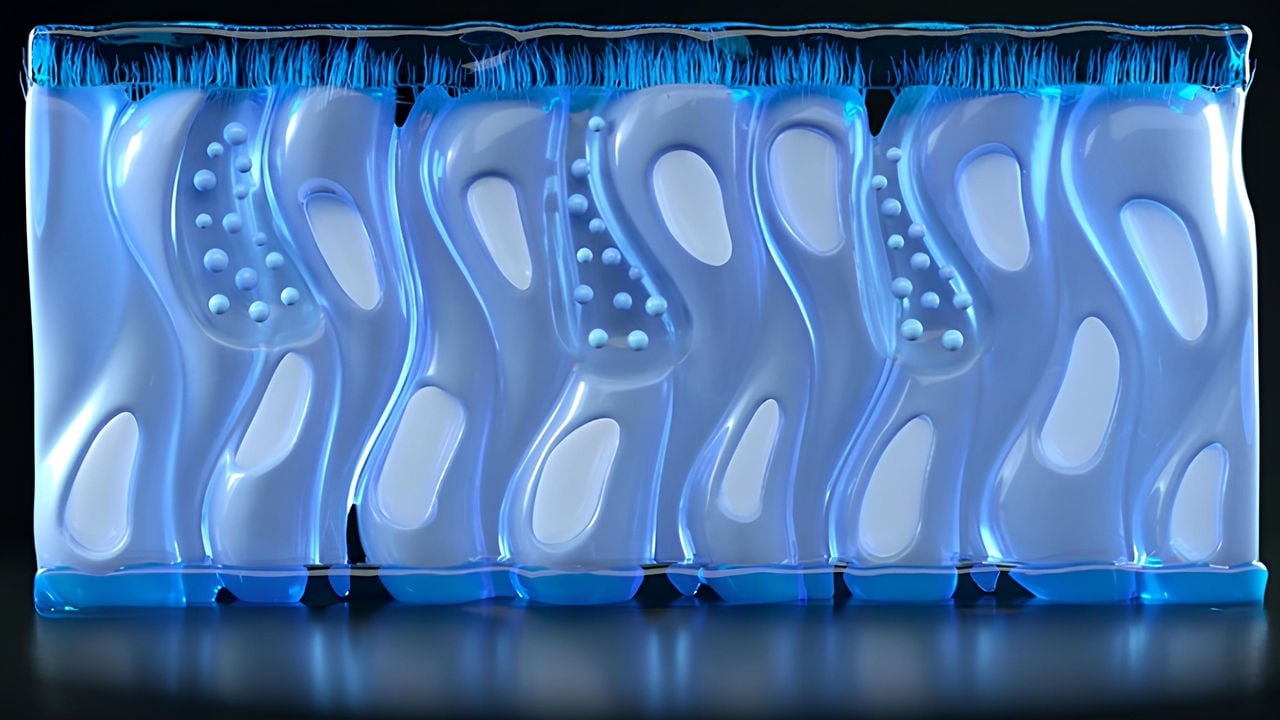
An artist’s rendering of the human bronchial cells used in a lung/liver-on-a-chip device. Such devices represent promising tools to reduce animal testing.
Despite this, there are still several hurdles to overcome before such technologies are implemented more widely in industry. A team of 46 experts, including PMI scientists, analyzed the current life cycle of organ-on-a-chip-based assays. The resulting report provides recommendations to enhance the development, qualification, and regulatory acceptance of organ-on-a-chip systems, as this may encourage the reduction of laboratory animal use in the drug development process.
Scientists can also minimize the use of animals in research when they have a good understanding of the mechanisms that lead to disease or a negative reaction to a chemical. AOPs are frameworks that map out the sequence of biological events from initial exposure to a substance to adverse or undesired effects.
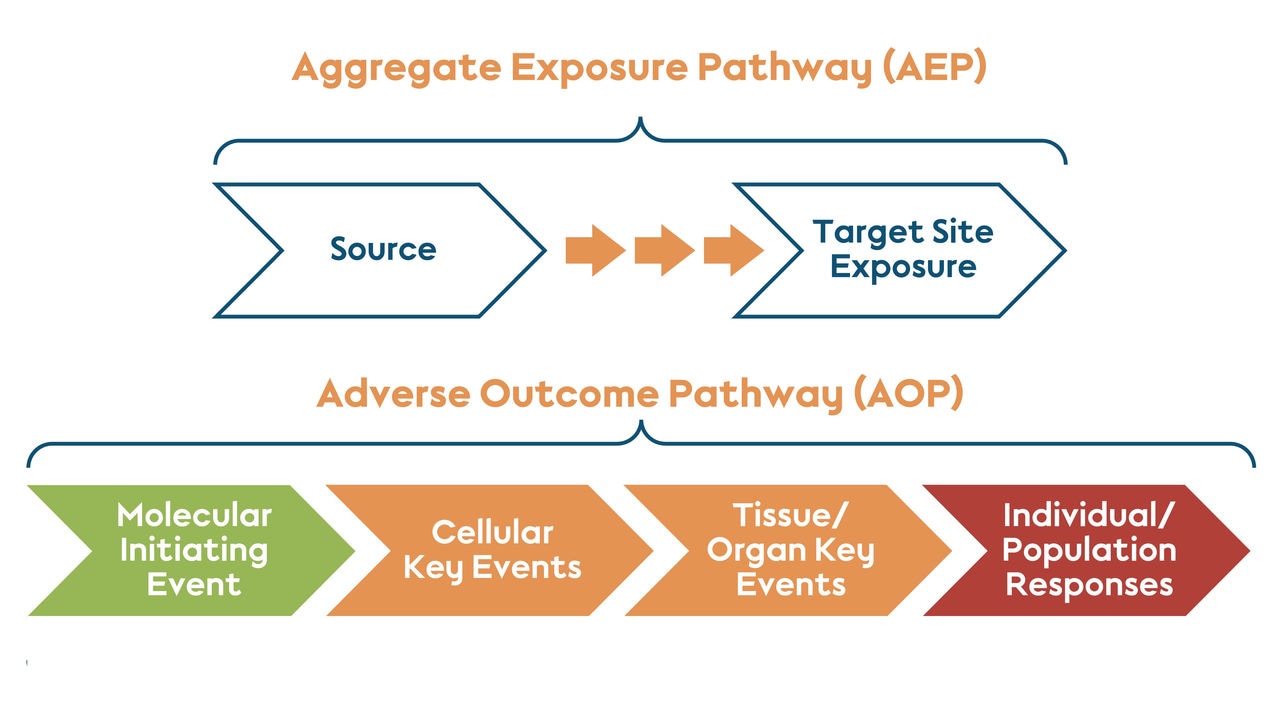
Establishing a pathway from exposure to response. An AOP begins when the exposure initiates the first event in the pathway. An AOP contains one or more key events inside the body, and ultimately results in the organism level or population level response.
For example, PMI scientists developed a quantitative AOP to study the risk of lung function decline caused by smoking. This model uses biological events like oxidative stress and impaired mucociliary clearance to predict potential improvements when switching to smoke-free products compared with continued smoking. Developing AOPs can help identify and address gaps in our understanding of disease development or chemical exposure. This allows scientists to identify key events involved in causing toxicity and adverse effects and to optimize nonanimal approaches for further investigations.
Artificial intelligence in research and product safety surveillance
At PMI, we use artificial intelligence (AI) to enhance the efficiency and accuracy of our scientific assessments and product development.
We have integrated AI with systems toxicology to predict the biological impacts of different compounds and refine risk evaluations accordingly. AI is particularly used in computational biology, where advanced models analyze vast datasets to evaluate adverse effects and biomarkers of exposure. We have also employed in silico techniques like the sbv IMPROVER platform, in partnership with IBM Research, which uses crowdsourcing and machine learning to validate toxicological models and methods. Such systems help us understand the impact of our smoke-free products on cellular and tissue models, thereby contributing to the development of less harmful products.
PMI has also embraced AI-based technologies to modernize safety surveillance of our smoke-free products. We have implemented ArisGlobal’s LifeSphere® Safety platform, which employs AI, machine learning and robotic process automation, to facilitate and improve safety surveillance of our products. By using this platform, we streamline lengthy, time-consuming processes, such as case management and risk detection, while delivering high-quality data. The system also enables real-time monitoring and enhances data accuracy. This allows our global medical safety team to maintain robust postmarket surveillance and ensures access to the most up-to-date safety profiles for our smoke-free products.
PMI’s broader scientific impact
Whether they are researching our smoke-free products or branching out to other topics, such as botanicals or respiratory drug delivery, our scientists apply state-of-the-art methods and standards for study development, data collection, and analysis. As is often the case in science, the approaches we’ve used and data we’ve collected throughout our assessment program can be useful to researchers beyond the tobacco industry. Our scientists are proud to be working toward delivering a smoke-free future, and it’s increasingly evident that, by sharing of our science, their efforts can have an even broader positive impact. That’s an opportunity we’re not willing to pass up.

Read the Scientific Update magazine
The Scientific Update magazine is focused on PMI's research and development efforts, milestone studies, industry regulations, and more. View the latest issue, or read the articles online.