Effects of switching to a heat-not-burn product on biomarkers of potential harm
What is PMI’s exposure response study?
The exposure response study is an important part of our product assessment of the THS. This study was designed to answer health-related questions about the THS as it is actually used by healthy adult smokers who switch to it. THS is an example of a heated tobacco product (HTP) which is a category of smoke-free products that, in general, heat the tobacco just enough to release a nicotine-containing aerosol without burning. Because tobacco is heated and not burned, there is no smoke, and the average levels of harmful and potentially harmful constituents (HPHCs) in the generated aerosols are significantly reduced compared with cigarette smoke. While these facts should be true for any HTP, they need to be confirmed scientifically on a product-by-product basis.
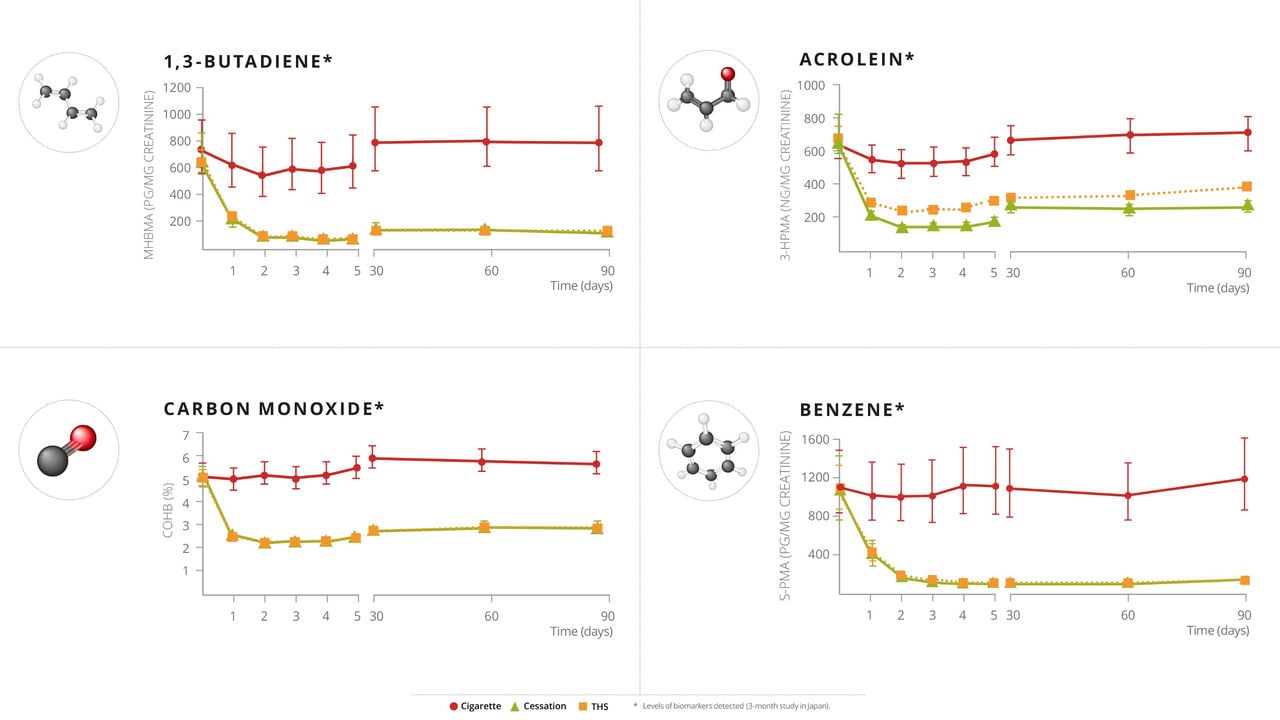
PMI has conducted multiple aerosol chemistry studies on THS, documenting approximately an average of 90-95% decrease in HPHC emissions in THS aerosol relative to cigarette smoke, as well as reduced exposure to HPHCs among participants who switched predominantly from cigarettes to THS versus those who continued to smoke. To understand if this reduced exposure is also associated with favorable changes in biomarkers of potential harm (BoPH), PMI researchers conducted an Exposure Response Study, which was published in Cancer Epidemiology, Biomarkers & Prevention, a journal of the American Association of Cancer Research.
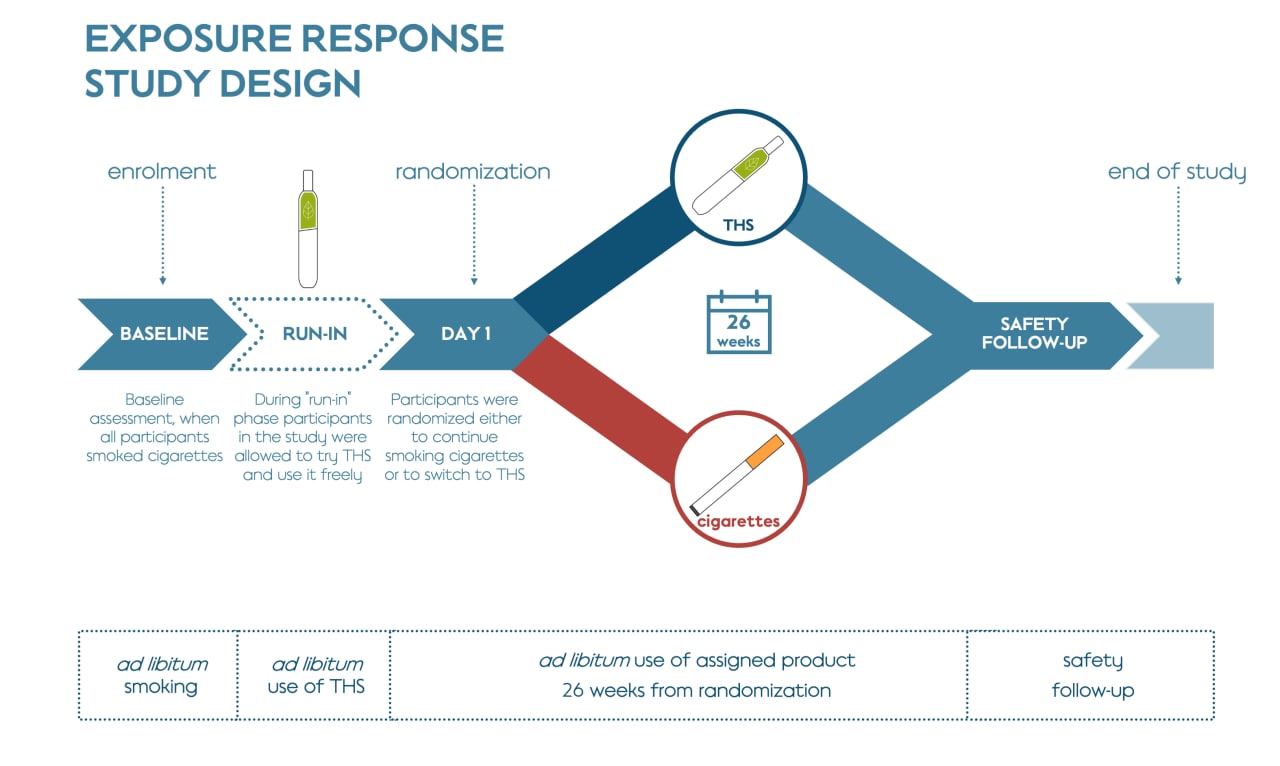
The 6-month clinical study was designed to verify if the reduction in exposure to HPHCs was associated with favorable changes in biological response (using BoPH as indicators of the biological response) in healthy adult smokers after entirely or predominantly switching to THS. It was followed by an extension study which evaluated sustained changes in the set of BoPH over a further 6 months.

The THS exposure response study, sometimes abbreviated ERS, was published online early in November 2019 in the journal Cancer Epidemiology, Biomarkers & Prevention. The study is also registered on clinicaltrials.gov under the identifier NCT02396381. The study’s 6-month extension, clinicaltrials.gov identifier NCT02649556, was published in June 2024 in the journal Biomarkers.
What are biomarkers?
Most changes in our health—for the better or for the worse—can be measured and quantified, but only if we know what signs to look for. Biomarkers are those signs. They are molecules that are usually (though not always) found in blood, other body fluids, or tissues, and they’re produced as a result or as part of processes in the body. Other measurements can be biomarkers as well, like a measurement of how much air a person can forcefully exhale in one second.
Here, Dr. Patrick Picavet briefly explains what biomarkers are.
Biomarkers measured in the initial 6-month exposure response study
Eight BoPH were selected to be the primary focus for the study. They were selected because they could serve as indicators of potential harm to the body (which is why they are called biomarkers of potential harm or BoPH) and because they’re known for improving within 6 to 12 months after smoking cessation. The publication also presented additional results on the effects of switching to THS on many other biomarkers, which you can learn more about by reading the publication.
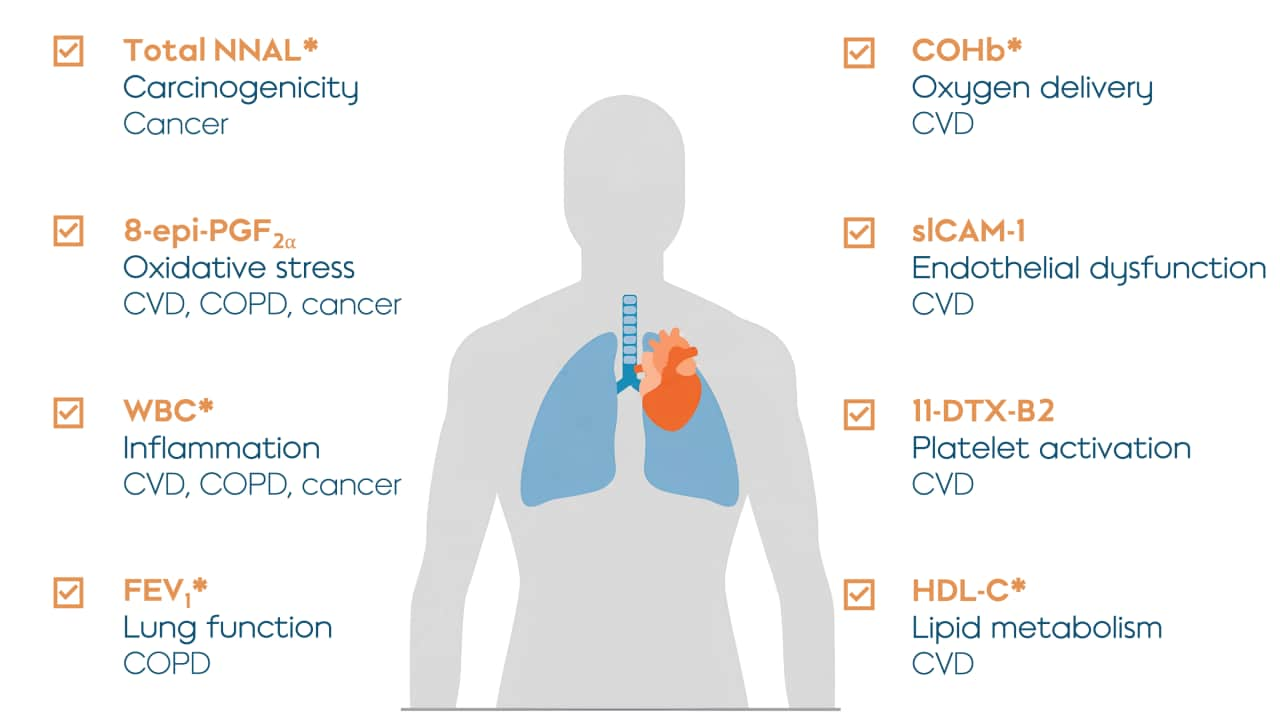
Eight BoPH were measured in the study. The abbreviated name of each biomarker is shown in orange; the biological function or change that the BoPH are related to are shown in bold; and the conditions or diseases associated with the biomarker is in plain text. Asterisks refer to five out of eight biomarkers that showed statistically significant changes in the initial study, indicating that THS caused the improvements shown in the study with 95% confidence. CVD = cardiovascular disease; COPD = chronic obstructive pulmonary disease.
Switching to THS use compared with continued smoking
All eight BoPH in this study showed favorable changes in the same direction as with smoking cessation, even though the THS group were still allowed up to 30% continued use of cigarettes. On average, replacing some cigarettes with THS provided some improvement, while predominantly switching to THS led to greater improvements compared with continued smoking. Completely switching to THS significantly reduces the potential risk of harm compared with continued smoking.
While all eight BoPH showed favorable changes, five out of the eight were statistically significant at 6 months after switching to THS compared with continued smoking. Overall, the results of this study confirm THS as an acceptable alternative to cigarettes for adult smokers, and based on the positive biological effects, that switching to THS likely presents less risk of harm than continued smoking.
How was the initial exposure response study conducted?
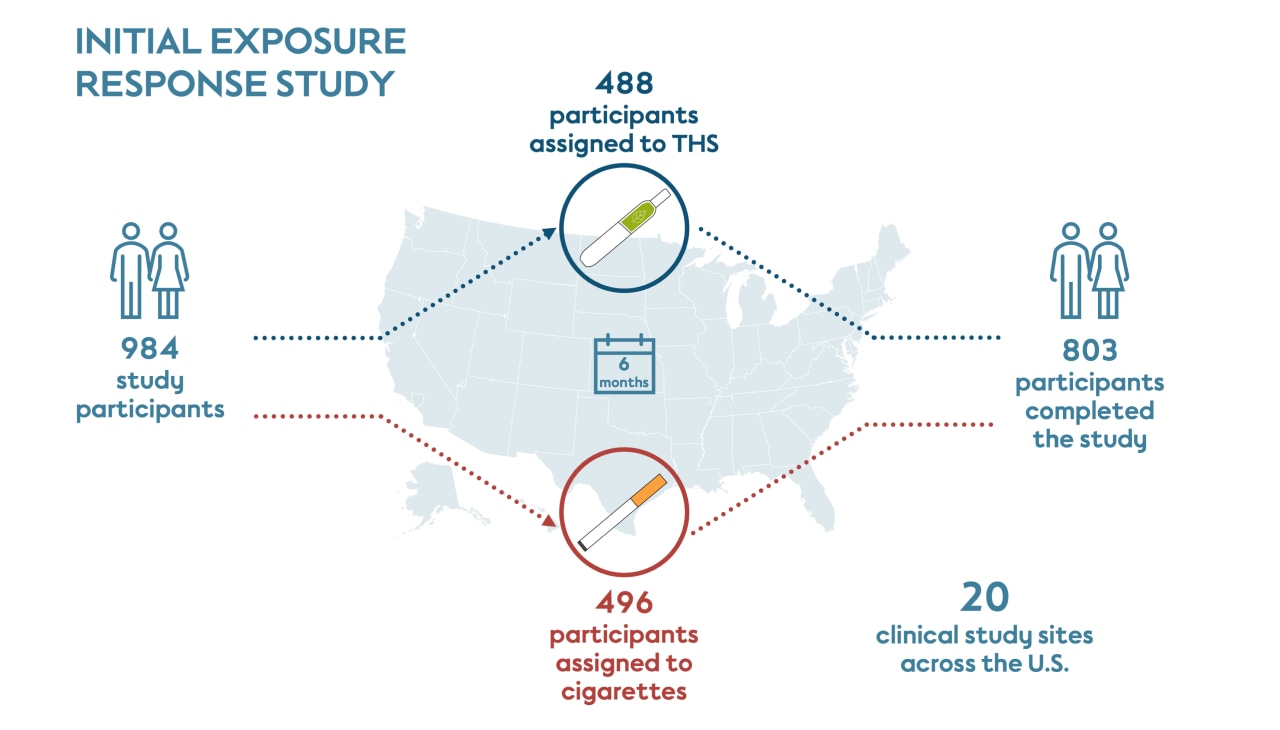
The initial 6-month exposure study was conducted at 20 study sites in the United States and managed by multiple independent contract research organizations. Recruitment started in March 2015, and the last subject completed the study in September 2016. Each clinical study site recruited their own participants after they received approval from an Independent Review Board.
This study included participants who had smoked at least 10 cigarettes a day for the past year, had been smoking for at least 10 years, and were not motivated to quit smoking within the 6 months of the study. Participants were randomly assigned to either continue smoking their own brand of cigarette (496 people) or to switch to THS (488 people) for the duration of the study.
For detailed inclusion and exclusion criteria, please refer to the clinical study protocol.
Participants who were assigned to switch to THS were provided with the product and told to use it exclusively while recording all tobacco products they used. The cigarette smoking group was asked to continue purchasing their preferred brand of cigarettes as usual. About half of the participants assigned to THS self-reported that they predominantly used THS for at least 70% of their tobacco use (cigarette and THS), which is termed “THS use” for this study.
To reflect the realistic use of THS, participants were classified into four groups, according to their product use pattern:
- Predominant THS use: using THS for ≥70% of daily use on average
- Dual use: 1% to <70% THS use
- Cigarette smoking (cig): <1% THS use
- Other use: participants who did not meet the above criteria
Exposure response study 6-month extension
Immediately following the initial study, a 6-month extension was conducted to monitor the changes to the eight BoPH between predominant THS users versus cigarette smokers over a longer period. This extension study aimed to determine:
- If the impact of THS use on the biological response was maintained over 12 months.
- To understand if people successfully continued to use THS over the longer period.
Out of the participants who completed the initial 6-month study, a total of 672 participants decided to continue to participate in the extension study, using the product they were randomized to in the initial study, for an additional 6 months.
What were the results from the extension study?
While a strict comparison between the initial study and the extension is not possible, because not all subjects of the initial study were enrolled in the extension, the results between the two studies are comparable for all BoPH.
For participants who predominantly used THS, the favorable biological responses observed at 6 months (and a reduction in the likelihood to cough, reported in the initial study as adverse events) were overall maintained at 12 months, compared to participants who continued smoking cigarettes. In this group, the data indicated an inverse response, so that the fewer cigarettes smoked per day in addition to THS, the greater the magnitude of beneficial effects among healthy participants.
For participants who used more than 30% cigarettes in addition to THS, if a favorable biological response was still observed, it was of much lower extent than the one observed in predominant users.
More about reduced risk
On July 7, 2020, the U.S. Food and Drug Administration (FDA) authorized the marketing of the IQOS tobacco heating system (the commercial brand of THS) as a modified risk tobacco product (MRTP) with reduced exposure information. IQOS is the first and only electronic nicotine-delivery product to be granted marketing orders through the FDA's MRTP process.
In particular, the agency determined PMI had demonstrated that because the IQOS system heats tobacco and does not burn it, it significantly reduces the production of HPHCs compared with cigarette smoke.
Findings from the two exposure response studies discussed here bring additional scientific evidence of the potential of THS to reduce the risks of some smoking-related harm in smokers who would otherwise continue to smoke cigarettes. Additional studies are however needed to evaluate disease risk associated with THS use. However, the best way for adult smokers to reduce the harm associated with smoking is to quit tobacco and nicotine altogether.