PMI plans clinical studies on the impact of switching from cigarettes to THS on smoking-related diseases
One study looks at chronic obstructive pulmonary disease (COPD), specifically in subjects who smoke and who have mild and moderate disease and history of chronic bronchitis. The study will investigate whether disease progression (measured by lung function and COPD symptoms) is slower for those smokers who switch from cigarettes to the Tobacco Heating System (THS) compared to those who continue to smoke cigarettes.
The purpose of the second study is to demonstrate whether improvements in flow mediated dilation (FMD) occur in participants who have one of two forms of cardiovascular disease (CVD, specifically coronary artery disease or peripheral arterial disease) after they switch to THS from cigarettes. Impaired FMD is a non-invasive predictive risk indicator of cardiovascular events.
Annie Heremans, PMI’s Chief Medical Officer emphasized the significance of these two clinical studies: “These are the first and most comprehensive clinical studies investigating whether switching to a smoke-free product will reduce the harm and risk of major tobacco related diseases.”
For people who smoke, the best choice they can make in the interest of their health is to quit using all tobacco- or nicotine-containing products altogether. For those who would otherwise continue smoking cigarettes, switching to a scientifically substantiated less harmful product such as THS, which doesn’t burn tobacco, is an alternative approach to reduce the harm caused by cigarette smoking.
So far, clinical studies conducted by PMI have followed healthy adult smokers who make the switch to smoke-free products as compared to smokers who continue to smoke cigarettes. These will be the first studies that seek to determine whether switching from cigarettes to THS can also slow the progression and improve symptoms of smoking-related diseases among smokers who have already developed COPD or CVD.
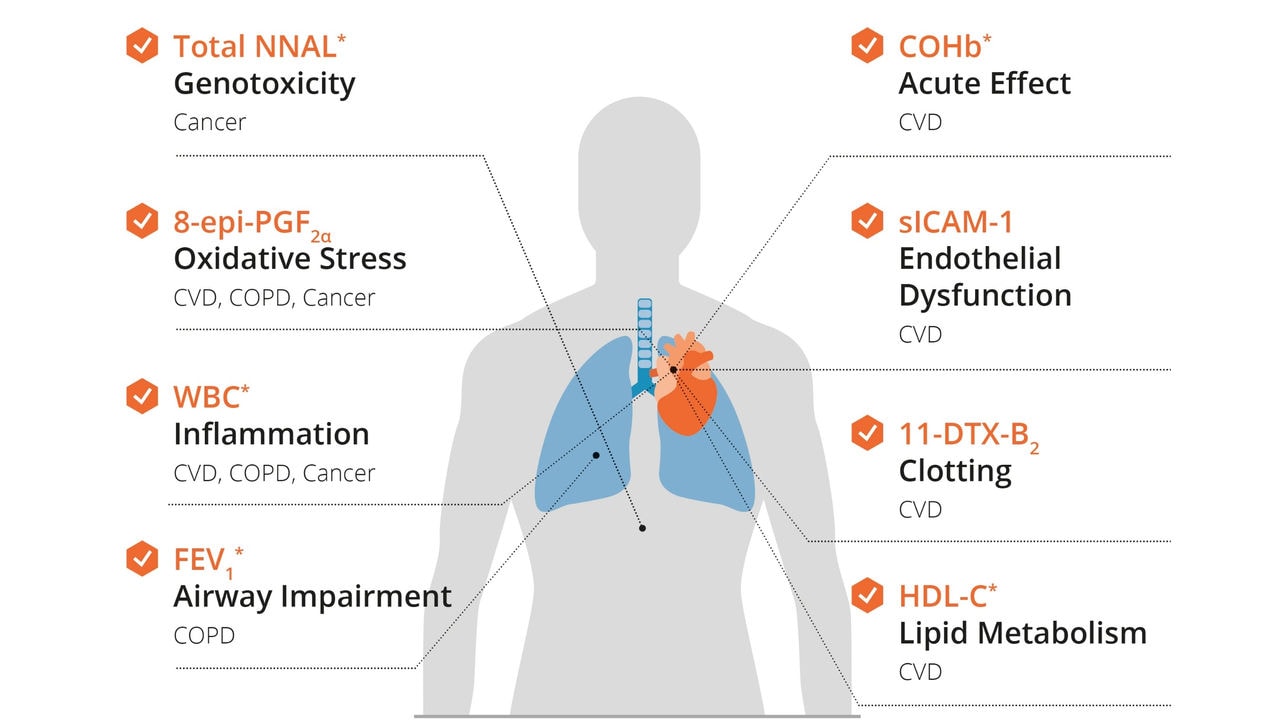
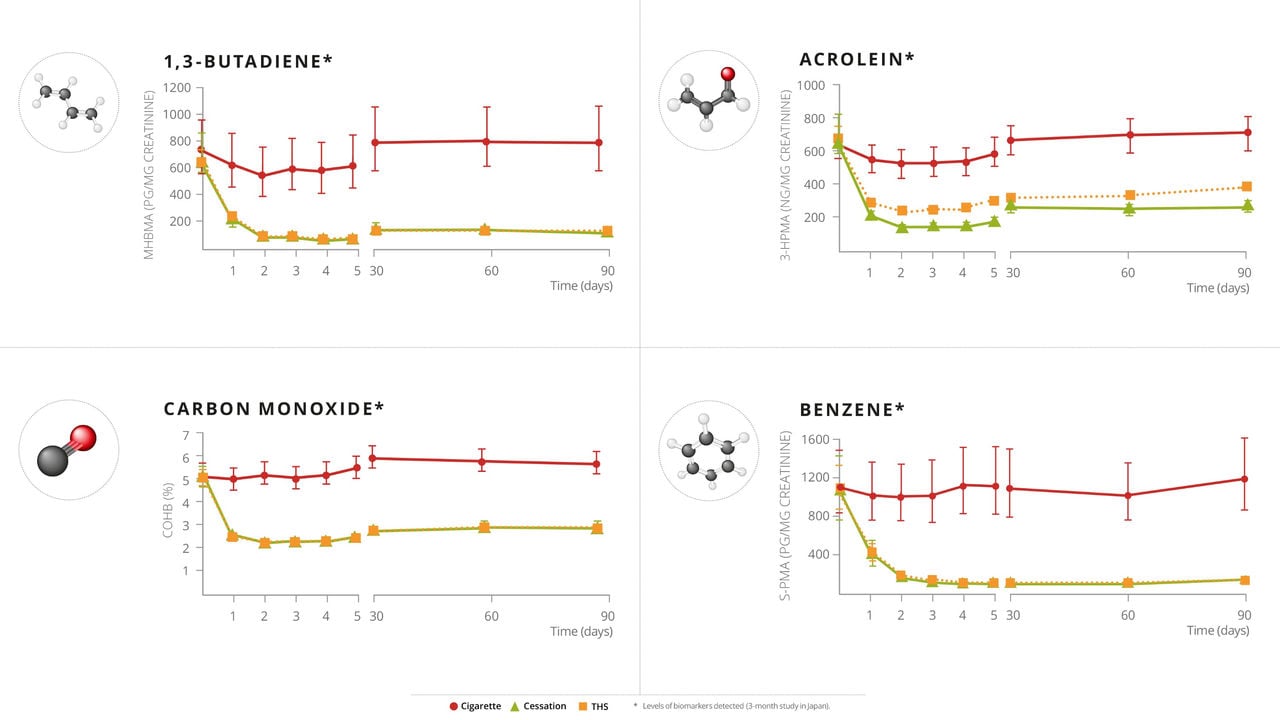
These will be the largest clinical studies ever conducted on THS. Each study will enroll approximately 1800 subjects, with 900 subjects switching to THS. For context, each of these studies involves roughly twice the number of participants as compared to our earlier Exposure Response Study, a clinical study in healthy volunteers conducted over 6 months and extended to 12 months, which showed improvements in eight out of eight biomarkers of potential harm with statistically significant improvements in five of those biomarkers.
“These are large multi-country studies, involving many clinical sites across the globe. Definitely a wider footprint compared to our earlier clinical studies. These studies are part of a bigger program, including an epidemiological study with healthy smokers and heated tobacco product users in countries where heated tobacco products are available.” said Barbara Pellegrini, Sr Manager Clinical Research Strategic Planning & Business Operations. She is the Program Leader for these two clinical studies.
Upcoming Research
The 3-year COPD study and the 1-year CVD study will be conducted in the United States as well as other countries in Europe and Asia. For both studies, current smokers who have the smoking-related diseases indicated in these studies will be split into three study groups. Smokers who are not willing to quit smoking at study entry will self-select to continue cigarette smoking, or to switch to THS use; smokers who are willing to quit at entry criteria will join the smoking abstinence group. The participants who are part of the THS arms will receive the most recently developed version of THS: THS 3.0 which is marketed as IQOS Iluma or IQOS 4.0, as well as induction tobacco sticks for the duration of the study.
As with all of PMI’s clinical studies, these two new clinical studies will be performed in accordance with the principles of the International Council for Harmonisation’s Good Clinical Practice (ICH GCP), and with ethical principles that have their origin in the Declaration of Helsinki. Prior to each study start, the clinical study protocol and other relevant documents will be subject to review and approval by an Independent Ethic Committee (IEC) and/or Independent Review Board (IRB) and will follow local regulations.
Details of the studies will be made available on clinicaltrials.gov.
In addition to the above described two clinical studies, and consistent with United States Food and Drug Administration’s (FDA) “Premarket Tobacco Product Applications and Recordkeeping Requirements Rule” and FDA’s “Premarket Tobacco Product Applications for Electronic Nicotine Delivery Systems”, PMI is also ready to initiate a prospective Actual Use study in the United States. This study is designed to provide information addressing how consumers actually use the product and how consumers understand instructions for use and safety warnings.
All Studies results will be submitted to multiple competent government and regulatory agencies, including the United States Food and Drug Administration.