What perception and behavior studies reveal: Tobacco and nicotine use in a changing world
Study 1 - How likely are U.S. adult smokers to switch to a novel e-vapor product?
PMI has conducted an actual use study to better understand whether an e-vapor product can have an impact on adult smokers' use patterns in settings close to real world, and to what extent these patterns would shift in favor of smoke-free product use. The results were presented at the 77th Tobacco Science Research Conference, held in September 2024, in Atlanta, Georgia, U.S. and at the CORESTA (Cooperation Center for Scientific Research Relative to Tobacco) Congress 2024, in Edinburgh, Scotland.
What were the goals of the study?
Conducted under actual use conditions over an 8-week observational period in 2022, our study investigated the ability of a novel e-vapor product (an electronic nicotine delivery system [ENDS], specifically, the P4M3 Generation 2.0 System, featuring nonrefillable, replaceable cartridges filled with nicotine-containing e-liquid) in helping adult smokers either reduce their cigarette consumption or completely transition away from cigarettes.
The study was designed to assess how likely adult smokers are to switch to the e-vapor product (including the proportion of participants who became exclusive users of the study product), and to assess the impact of the product availability on cigarette consumption.
Previous studies have indicated that adult smokers who switch completely from cigarettes to e-vapor products, or reduce their cigarette consumption by 50% or more, may reduce their exposure to the harmful and potentially harmful constituents (HPHCs) present in tobacco smoke. In addition, findings from cohort studies and randomized controlled trials, summarized in the recent Cochrane Review, demonstrate that adult smokers who use e-vapor products exhibit higher rates of smoking reduction and higher rates of stopping or abandoning smoking than those who use cessation methods.
How was the study conducted?
This multi-site prospective study was conducted among existing daily adult smokers in the U.S., where research has indicated that switching to e-vapor products is the most common method used when attempting to stop smoking. Both exclusive cigarette smokers and dual users of cigarettes and e-vapor products were recruited.
A total of 821 U.S. daily adult smokers were enrolled in the 10-week study, with 720 included in the final analytical sample (353 exclusive cigarette smokers and 367 dual users). Participants were randomly selected from consumer databases and sampled to obtain a study population that represents the U.S. adult smoking population in terms of sex, age, and race.
The study product was available ad libitum in two variants (tobacco and menthol), with a single nicotine concentration (3.5%). The study design was based on previously conducted actual use studies and took place in three phases:
- A 1-week baseline phase to assess cigarette and other TNP use prior to the introduction of the study product.
- An 8-week observational phase where participants were provided the study product and were followed to assess study product use and changes in cigarette and other TNP use.
- A 1-week closeout phase for continued surveillance of potential adverse events. No new safety concerns related to the study product emerged during this study.
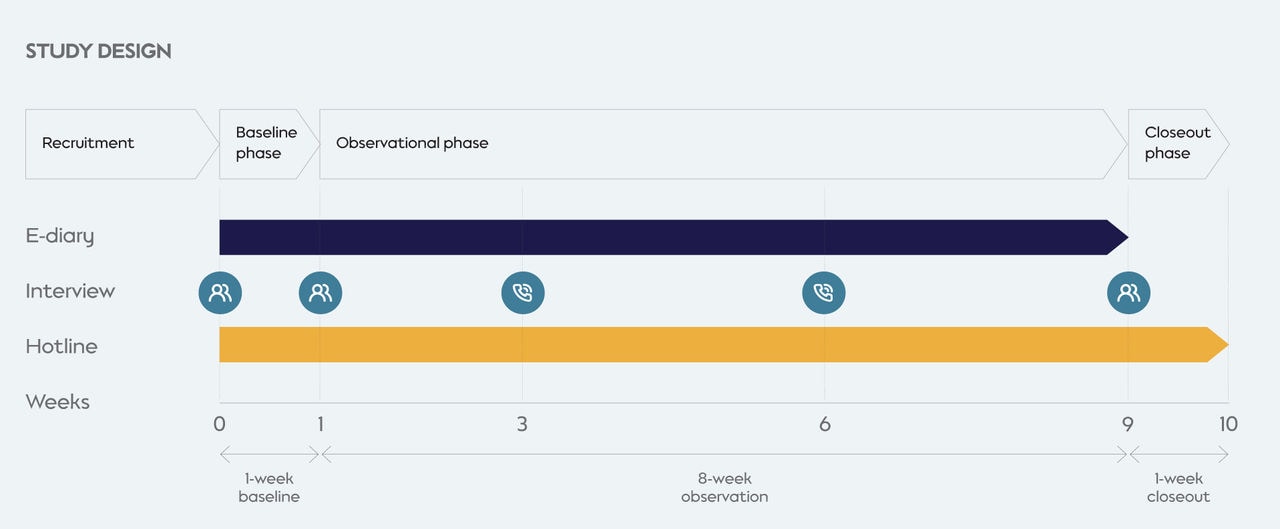
Participants self-reported their daily use of cigarettes and other TNP use (during the baseline and observational phases) and the study product (during the observational phase) in an app-based e-diary. Participants were also interviewed following weeks 2, 5, and 8 of the observational phase, to evaluate sensory attributes of the study product.
Outcomes for actual product use were assessed by comparing results between the end of the observational phase and baseline. This included:
- The proportion of participants who exclusively used (i.e., switched completely to) the study product.
- The absolute change in cigarettes smoked per day.
- The proportion of participants who reduced cigarettes smoked per day by 50% or more.
- The proportion of participants who transitioned from daily to nondaily cigarette smoking.
Each of the outcomes was assessed separately for exclusive cigarette smokers and dual users and was stratified by the variant of the study product (tobacco, menthol, or both) used in week 8.
What were the results of the study?
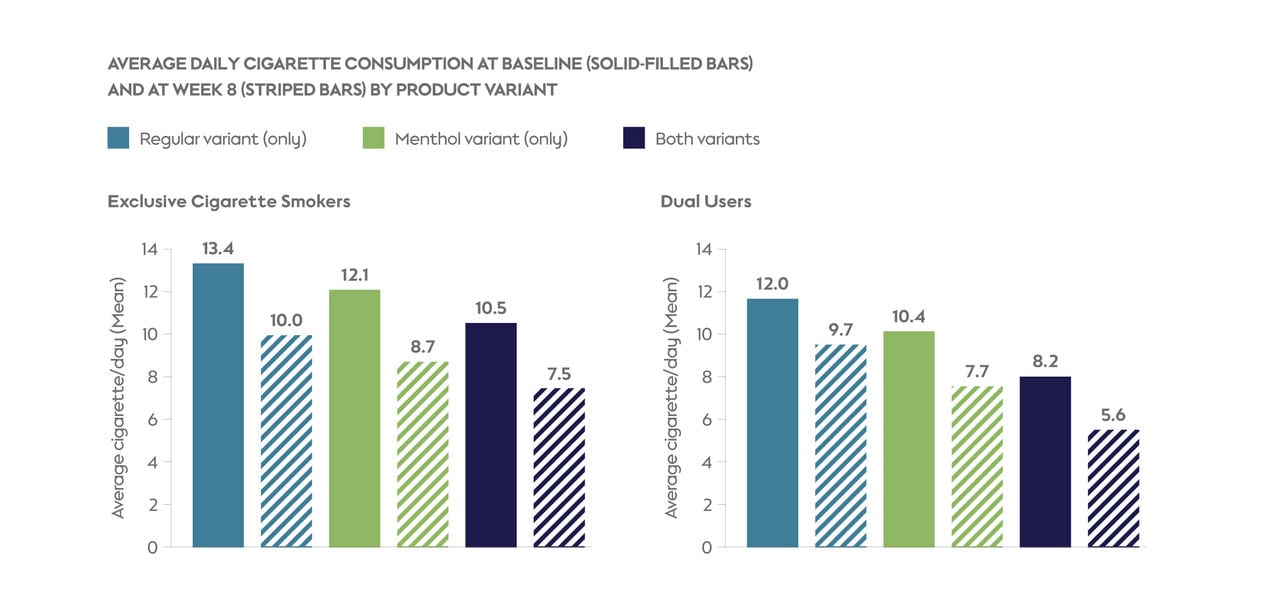
During the baseline phase, exclusive cigarette smokers and dual users smoked, on average, 13 and 12 cigarettes per day, respectively. At the end of the observational period (week 8), 86.2% of exclusive cigarette smokers and 92.1% of dual users used the study product, with 4.0% of exclusive cigarette smokers and 4.1% of dual users having switched completely. Dual users used the study product more often (mean: 7.3 times per day) than exclusive cigarette smokers (5.0 times per day).
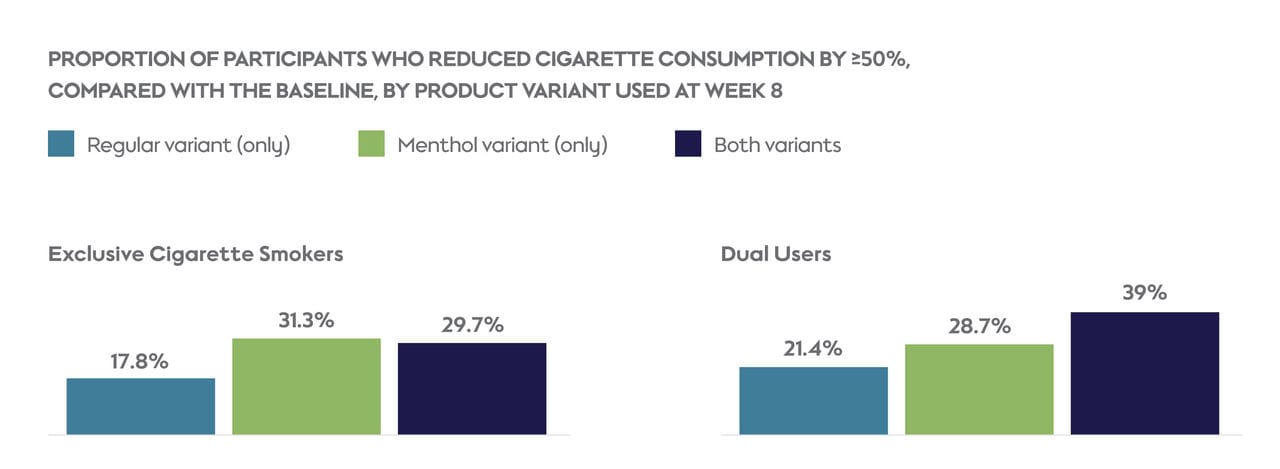
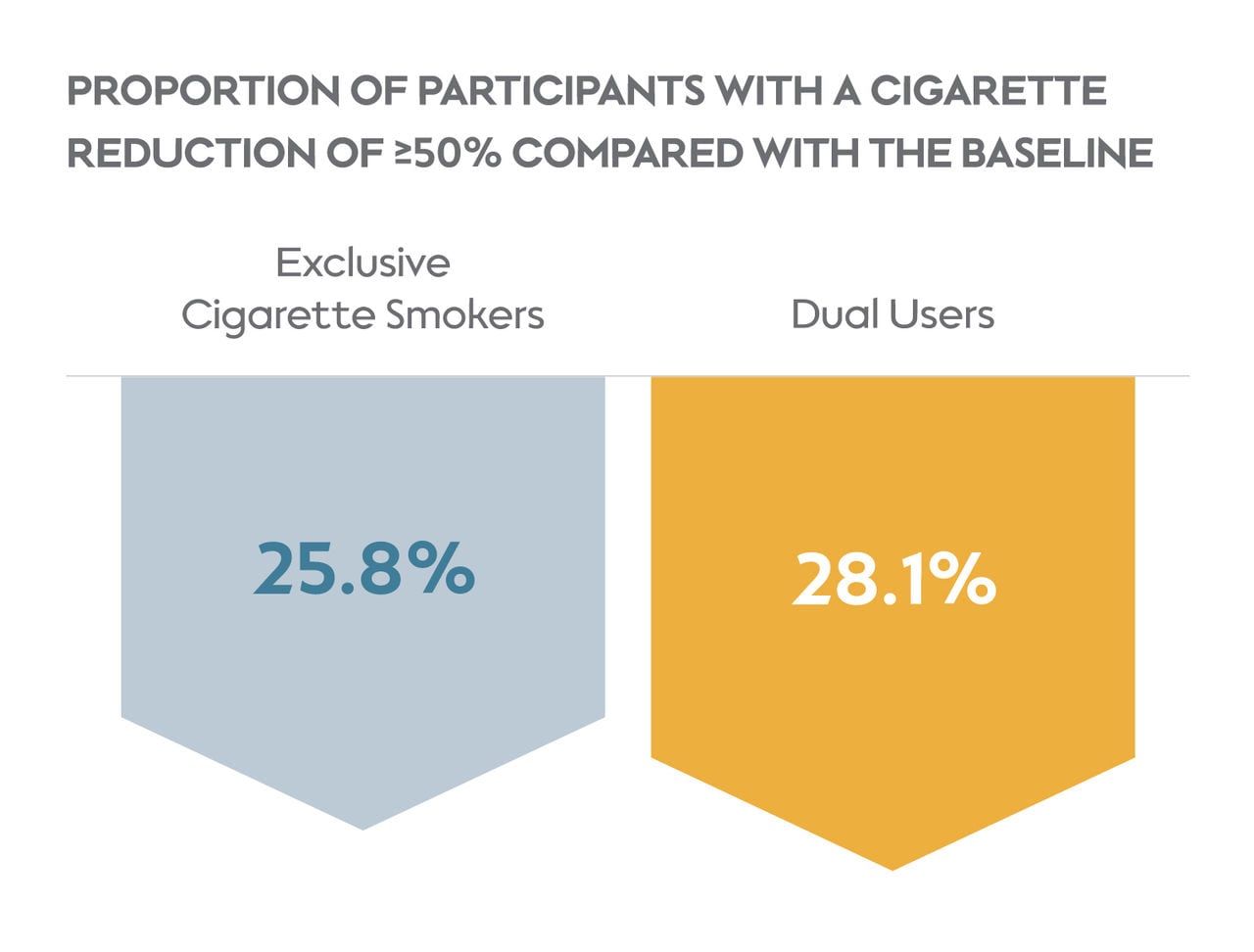
Overall, participants in both study groups reduced their daily cigarette consumption from baseline to week 8, with 25.8% of exclusive cigarette smokers and 28.1% of dual users reducing their cigarettes smoked per day by 50% or more.
At week 8, more exclusive cigarette smokers (54.6%) and dual users (49.4%) used only the menthol variant than used only the regular variant (33.2% and 33.1%, respectively). At the same time, 12.2% of exclusive cigarette smokers and 17.5% dual users used both variants.
There was also a reduction in the proportion of participants who smoked daily at week 8 compared with baseline. At week 8, 87.1% of exclusive smokers smoked daily, compared with 98.3% at baseline. For dual users, there was a reduction from 91.8% smoking cigarettes daily at the start to 79% smoking daily at week 8.
In both study groups, the proportion of participants who reduced their cigarette consumption by ≥50 was higher among participants who used menthol or both variants of the study product, compared with those who used only the regular variant.
Do the study results indicate that smoke-free products are an acceptable alternative for cigarette smokers who don’t quit?
This study focused on use patterns of an e-vapor product among adult cigarette smokers who have little or no prior experience of using smoke-free products, or who are dual users of cigarettes and smoke-free products. These groups are the intended users of the study product.
The results indicated that the study product was an acceptable alternative to both current adult cigarette smokers and dual users with a large proportion of subjects using the study product after 8 weeks (some of whom switched completely).
Over a quarter of subjects in each group had reduced their cigarette consumption by ≥50%.
The study also showed that participants had a greater average liking (in terms of smell, taste, and aftertaste) for the menthol variant than the regular variant. A high proportion of participants (in both subject groups) who used the menthol variant or both variants exclusively had reduced cigarette consumption by >50% at week 8 than the regular variant alone. These results suggest that the availability of menthol e-vapor product variants may be a factor in the number of cigarette smokers or dual users who reduce their consumption or switch completely to e-vapor products.
The study did have limitations, including: participants did not have to pay for the e-vapor product but did have to pay for any other existing tobacco product they used; use of all products was based on self-reported measures; the e-vapor product was only available in two variants, which may have affected existing users of smoke-free products who were used to a wider range of flavors.
Overall, the results support existing evidence that e-vapor products, including the one used in this study, are an acceptable alternative to cigarettes for existing adult smokers who don’t quit and so may aid in reducing the harm from cigarette use.
Study 2 - Spotlight on users of the Tobacco Heating System (THS) in the U.S.
Investigators from Altria and PMI explored the profiles of users of THS in the U.S., shedding light on their tobacco use behaviors and perceptions of health risks compared with cigarettes. This research contributes to the growing body of evidence on the role of heated tobacco products (HTPs) in tobacco harm reduction.
Why was this study conducted?
THs produces aerosol with on average 95% lower levels of harmful and potentially harmful constituents (HPHC) compared with cigarettes, based on the World Health Organization (WHO) list of nine priority HPHCs mandated for lowering in cigarette smoke. In clinical studies, subjects who switched from cigarettes to THS had favorable differences in biomarkers of potential harm (BoPH) linked to smoking-related diseases compared with those who continued to smoke. For adult smokers who do not quit, THS may reduce the risk of harm to their health compared with continued smoking.
A recent cross-sectional study delves into the tobacco use behaviors and perceptions of relative health risks among U.S. consumers of THS. This survey-based study involved 688 adult THS users (aged 21 years or older) who had used at least 100 heated tobacco units (HTUs) with THS. Participants were recruited through PMI’s consumer database and completed a detailed online questionnaire between September 15 and November 14, 2021, to assess their sociodemographic characteristics, tobacco use history, current smoking behaviors, and perceptions of health risks associated with THS compared with cigarettes. The survey included both closed and open-ended questions to capture a comprehensive picture of users’ experiences and attitudes.
Sociodemographic characteristics of THS users in the study
Most of the participants were middle-aged (mean age 45 years), non-Hispanic White (73%) males (62%). Almost all had a history of smoking cigarettes—20 years on average before trying THS. Most (82%) had never tried a smoking cessation treatment or had not used one in the past year. This demographic supports the notion that THS may be used predominantly by established long-term adult smokers who don’t quit rather than unintended audiences.
Tobacco use patterns: A shift in behavior
On average, participants had used THS for just over 1 year, with many showing notable changes in their tobacco use habits. Just over half of participants (51%) were not smoking cigarettes at all at the time of the survey. Even among those who still smoked both cigarettes and THS, 84% reported smoking fewer cigarettes than before. This shift in behavior highlights the potential of THS to help adult smokers switch away from cigarettes completely or reduce their cigarette consumption.
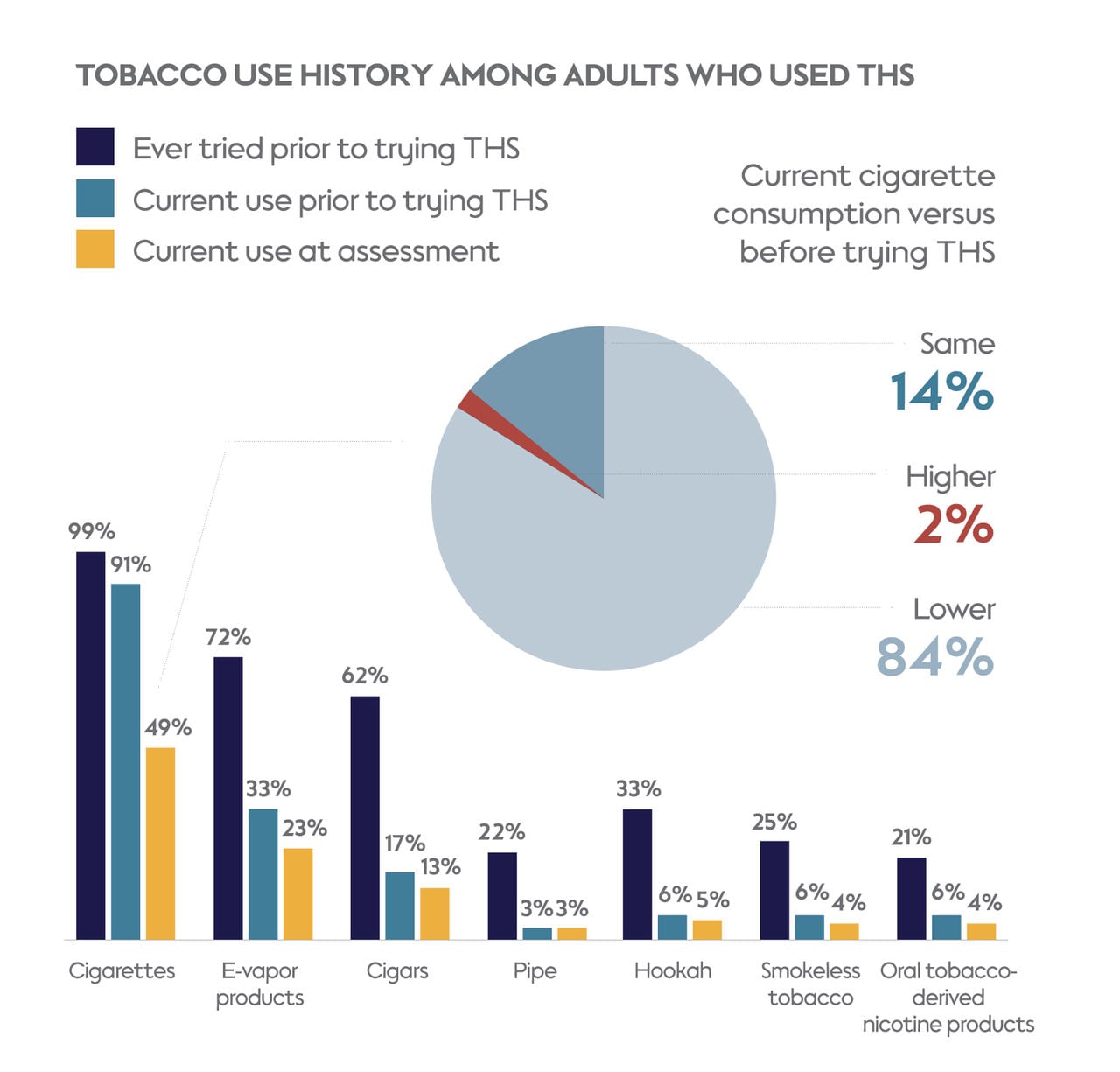
Tobacco use among study participants (N=688) before first trying THS and at time of assessment. Current use was defined as using a product ‘every day’ or ‘some days’ in response to the question “Do you now use/smoke … every day, some days, or not at all?”
Understanding THS daily use patterns
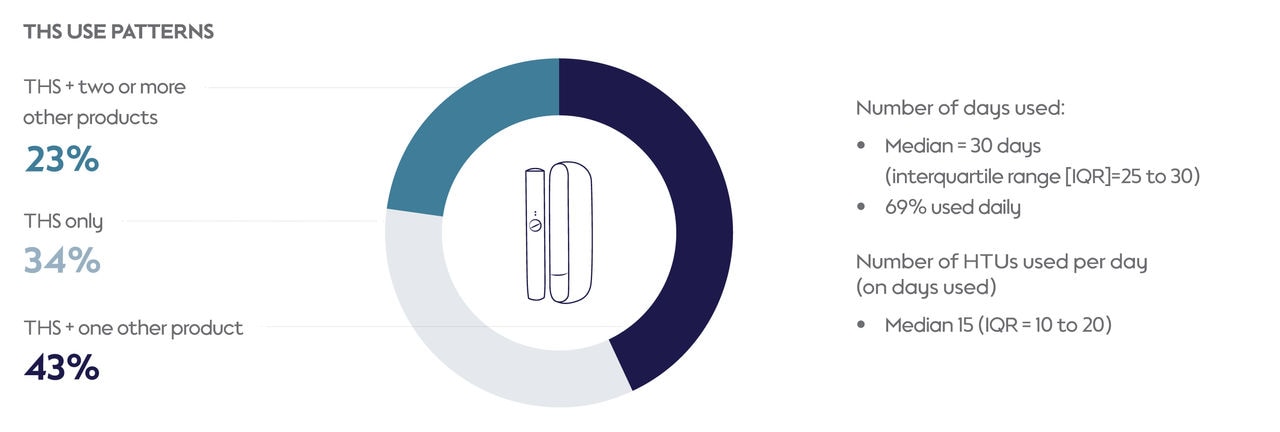
A substantial portion (69%) of participants who were using THS at the time of assessment reported using it daily in the month before the assessment. Those who had used THS for over 1 year were more likely to use it daily compared with those who had used it for 1 year or less (73% versus 65%; P=0.04).
On the days they used THS, participants typically used 15 HTUs. THS users who previously smoked menthol cigarettes tended to prefer menthol HTUs (93%), while those who smoked nonmenthol cigarettes leaned towards nonmenthol HTUs (74%). However, a sizable percentage of participants who currently smoked nonmenthol cigarettes preferred menthol HTUs when using THS. This highlights the potential role menthol varieties can play in encouraging adult smokers to switch to THS use and away from cigarettes.
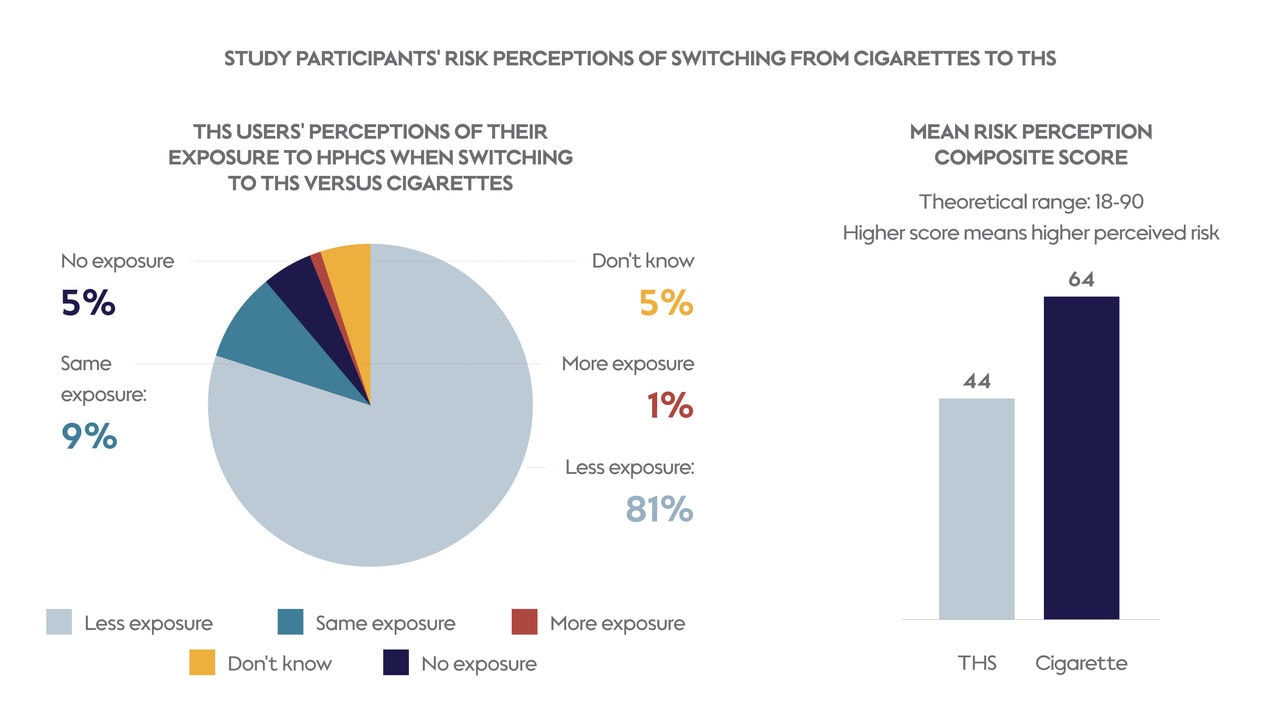
Perceptions of the relative risks of THS
One of the most compelling findings was how participants perceived the health risks associated with THS compared with cigarettes. A vast majority (81%) of participants understood the MRTP information authorized for THS by the U.S. FDA. In addition, 85% of this 81% understood that smokers must stop smoking completely and only use THS to reduce their exposure to HPHCs. Participants also indicated that they perceived THS as less harmful than smoking cigarettes. This perception of relative health risk is crucial in encouraging adult smokers who don’t quit to make the switch to THS or other smoke-free alternatives that are scientifically substantiated to be less harmful than cigarettes.
What are the limitations of this study?
Limitations of the study include its cross-sectional design, which limits causal interpretations of switching patterns or long-term health outcomes. It is also based on self-reported data, which may be subject to recall bias. In addition, the study primarily focuses on THS users in specific U.S. regions and may, therefore, not represent broader national or international patterns.
What are the implications of these findings for THS?
This study underscores the potential of THS as a harm reduction tool for adult smokers who don’t quit. The results provide supportive evidence for the potential of THS to help users switch from smoking by comprehensively assessing use behaviors and risk perceptions of THS users in a real-world setting in the U.S. The study also highlights the importance of accurate communication and education efforts to ensure that smokers are well informed about the benefits and risks of switching to smoke-free alternatives.
In the future, population-based research will be crucial to fully understand the potential public health implications of THS use across countries, including the U.S.
The bigger picture
Perception and behavior studies are more than just data; they offer a glimpse into the lives of users of smoke-free products—who uses them, how they use them, how/whether they use other TNPs in conjunction, and how they perceive the relative health risks of these alternatives. Studies like these show that people who use scientifically substantiated smoke-free alternatives tend to reduce their cigarette consumption or even stop their cigarette consumption altogether. They also underscore the importance of providing accurate information and education about smoke-free alternatives for adult smokers. By understanding the experiences and risk perceptions of users of smoke-free products, we can better support adult smokers who don’t quit in their transition to less harmful alternatives to cigarettes.

Read the Scientific Update magazine
The Scientific Update magazine is focused on PMI's research and development efforts, milestone studies, industry regulations, and more. View the latest issue, or read the articles online.




